Patient Position and Incision
For patient safety throughout a prolonged surgical procedure, careful positioning is required. We advocate the lithotomy position with open legs, thighs flexed at 15° on the abdomen and legs flexed 30° on the thighs (see Fig. 11.1, chapter 11). The legs are supported with St. Mark leg holders (AMSCO, Erie, PA) surrounded by alternating pressure devices (SCB Compression Boots, Kendall, Boston, MA), and protected from decubitus lesions by egg crate foam padding. The weight of the lower extremity is on the heel and not on the calf or popliteal crease.
The incision must be from the xiphoid to symphysis pubis; the xiphoid is excised. When combined with a self-retaining retractor (Thompson Surgical Instruments, Traverse City, MI), such an incision allows access and free continuous visualization of the abdominal and pelvic cavity (see Fig. 11.2, chapter 11). This exposure of the operative field facilitates complete lysis of adhesions and thorough peritoneal cavity exploration. In reoperative surgery the xiphoid to pubic incision includesawide excision of the prior surgical scar from the skin to the peritoneum, including the umbilicus. Unless there is a wide resection of prior incisions many recurrences take place in the prior incision.3 For this reason old incisions, as opposed to new incisions, are preferred.
 Lysis of Adhesions, Exploration, and Retraction
Lysis of Adhesions, Exploration, and Retraction
Currently, a major proportion of treatment for peritoneal surface malignancy is directed at recurrent disease. This timing should change as therapeutic strategies to prevent carcinomatosis and sarcomatosis become accepted and surgeons definitively interrupt further progression of primary disease presenting with seeding. In the patients operated in a recurrent disease setting, adhesions are a common problem during re-operative intervention. Adhesions alter the normal anatomy and prevent adequate exploration required to assess the extent of disease. Therefore, complete lysis of adhesions is the necessary initial dissection of the abdomen and pelvis. When entering the abdominal cavity, elevation of the abdominal wall using strong traction from skin hooks will minimize the possibility of damage to small bowel loops adherent to the abdominal incision (Fig. 13.1). Care must be taken to preserve the rectus abdominis muscles. These muscles will be needed postoperatively for patient mobilization and respiratory function. However, sacrifice of the posterior rectus sheath may facilitate a more rapid entrance beyond the scar associated with the prior incision. The abdominal wall must be cleared of viscera for a distance of 7–10 cm from the edges of the abdominal incision; following this the self-retaining retractor is inserted
and will facilitate enterolysis. Compression of tissues between thumb and index finger will thin out abdominal adhesions between bowel and abdominal wall or between bowel loops. This compression is a form of blunt dissection that can avoid damage to intestinal structures by adding tactile sensation to visualization in order to separate loops of intestine (Fig. 13.2).
As a requirement of complete exploration, resection of the gastrohepatic and gastrocolic ligaments must be performed. Exploration of the omental bursa is not possible unless it is widely opened.
Seromuscular tears are frequent during dissection in patients with many adhesions. These tears are marked with a single stitch and repaired definitely after intraoperative chemotherapy has been completed. Since adhesions in progressive abdominal-pelvic cancer are likely to contain tumor foci, the fibrous tissue is not merely divided but is resected and then collected for histopathologic examination. This complete clearance of adhesions is essential not only for a proper decision regarding the surgical procedure but also for the uniform distribution of intraperitoneal chemotherapy.
There are great advantages for mechanical retraction in performing peritonectomy procedures. The Thompson self-retaining retractor is the most useful and versatile instrument (see Fig. 11.2, Chapter 11). It allows multiple angles of retraction to be applied at variable strengths on the abdominal wall. Also, a second tier of retraction to remove viscera from an operative site can greatly facilitate dissection or suturing. The major function of the self-retaining retractor is a sustained complete visualization of the entire operative field that allows for centripetal dissection.
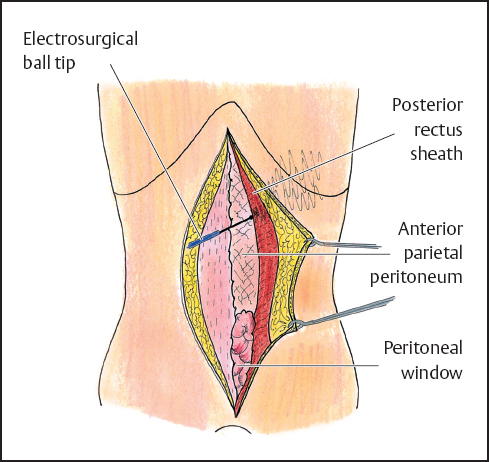
Fig. 13.1 Abdominal wall under tension to allow lysis of adhesions between the abdominal wall and the underlying bowel and preservation of the rectus abdominis muscle.

Fig. 13.2 The lysis and resection of intestinal adhesions requires finger and ball-tip electrosurgical dissection, irrigation, and smoke evacuation.
 Electrosurgical Dissection
Electrosurgical Dissection
After division of the skin with a scalpel, all subsequent dissection is performed with electroevaporative surgery.4 This methodology requires a ball-tipped electro-surgical handpiece. The preferred tip diameter is 0.3 cm and the tip is extended 10 cm beyond the hand control device (Fig. 13.3). The ball-tip is powered by a high-voltage electrosurgical generator. For dissection one commonly uses maximal pure cut. The pure-cut mode electroevaporizes the tissues that are in contact; this eliminates the accumulation of tissue debris as occurs with coagulation current. The buildup of coagulated tissue and distortion of normal anatomy obscures visualization of the dissection site. Because of the high voltage, the electroevaporation of tissue produces a copious amount of smoke that interferes with visualization of the operative field. The smoke is an important health hazard that needs to be eliminated from the operating room environment. To evacuate the smoke, a laser-smoke evacuator is necessary for electroevaporative surgery.
In addition to excess smoke production requiring a smoke vacuum, to prevent heat damage, frequent intermittent saline irrigation at the site of tissue dissection is necessary. Heat necrosis is of great value at the margin of dissection to minimize disease progression. Heat necrosis of a tubular structure can cause fistula formation that results in excessive morbidity and mortality. Frequent saline cooling is essential.
A major advantage of ball-tip electrosurgical dissection is the creation of a lens-shaped (lenticular) defect as a result of the electroevaporation of the tissue (Fig. 13.4). If strong traction is maintained at the plane of dissection, visualization of vital structures occurs before injury occurs. In contrast, the defect created by a blade-tip is linear so that a visual monitor of the structures at the deepest extent of the dissection is not possible. Consequently the risk of damage to vital structures is much greater with blade dissection than with ball-tip dissection.
Long-term local control of peritoneal implants as a result of ball-tip electrosurgical dissection is related to the growth pattern of the tumor. Adenocarcinoma is an infiltrative process and electrosurgery may only remove the superficial portion of the tumor mass. Although small implants are minimally invasive, deeper resection of large adenocarcinoma implants can provoke severe damage to the underlying structures. In contrast cystadenocarcinoma (adenomucinosis) or low-grade sarcomas may have an expansive growth with minimal invasion of the underlying tissues. The ball-tip dissection can resect the tumor mass leaving behind little or no residual tumor. The long-term success of intraperitoneal chemotherapy in maintaining control of peritoneal surface malignancy may depend on the mass of residual malignancy that remains following cytoreduction. Microscopic residual disease is reliably controlled by intraperitoneal chemotherapy; control of macroscopic or gross residual disease is not usually maintained (Fig. 13.5).
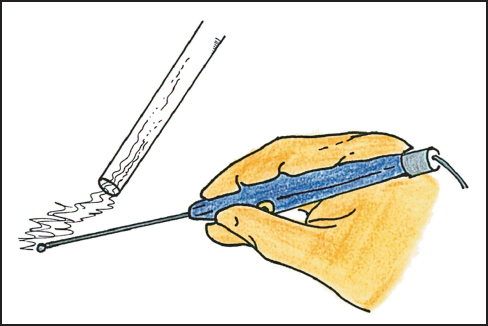
Fig. 13.3 Ball-tip electrosurgical device.
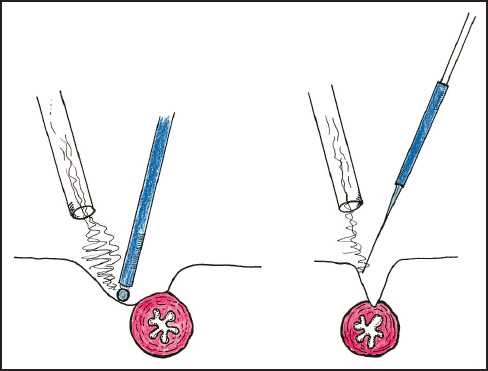
Fig. 13.4 Comparison of the operative defect created by ball-tip and blade-tip electrosurgery.
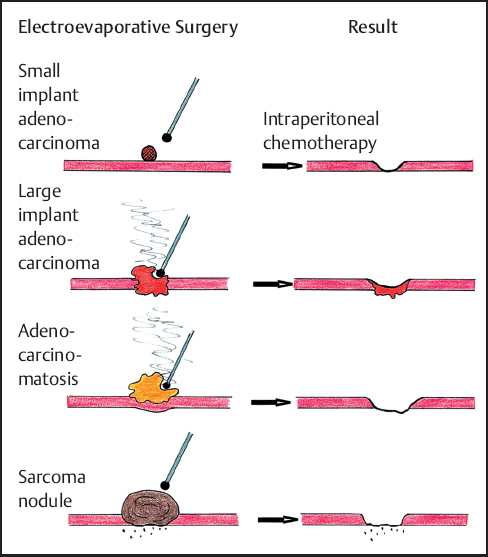
Fig. 13.5 Long-term local control expected after ball-tip elecrosurgical dissection and perioperativeintraperitonealchemotherapy with different peritoneal surface tumors.
During dissection pure-cut electroevaporative surgery provides absolute hemostasis of small vessels up to 1.0 mm in diameter. Coagulation current can control vessels from 1.0 to 3.0 mm in diameter. Electrosurgery is most effective in providing hemostasis if the vessel is compressed by upward traction by the index finger of the nondominant hand. The vessel walls are reliably sealed together if the blood has been eliminated from within the lumen. Larger vessels are first visualized by electrosurgical dissection, and then clearly defined from surrounding fat by “thumb and index finger compression.” Once clearly visualized the larger vessels are ligated in continuity and divided. The same technique is also applied to large lymphatic vessels. Absolute hemostasis and lymphostasis combined with frequent large volume irrigation and suction are responsible for a clean operative field and clear visualization of all vital structures. The translucent nature of the tissues should be maintained by eliminating blood from the operative field. The result should be minimal blood loss and no disruption of cancerous masses as the centripetal dissection proceeds.
In some instances a generous free margin of dissection is not possible in performing peritonectomy. Ball-tip electrosurgical dissection destroys tumor foci encountered at the interface of cancerous peritoneum and normal tissue during peritonectomy by causing a heat necrosislayerupto 1.0 mm in thickness from the dissection margins. This margin of heat necrosis provides a small but usually adequate tumor free margin that often results in curative peritonectomy (Fig. 13.6a–c). If narrow margins of dissection are attempted using scissor or knife dissection the margins of the peritonectomy are extensively tumor contaminated so that perioperative intraperitoneal chemotherapy is less likely to eradicate residual cancer cells. Peritonectomy can only be maximally effective using electroevaporative surgery.
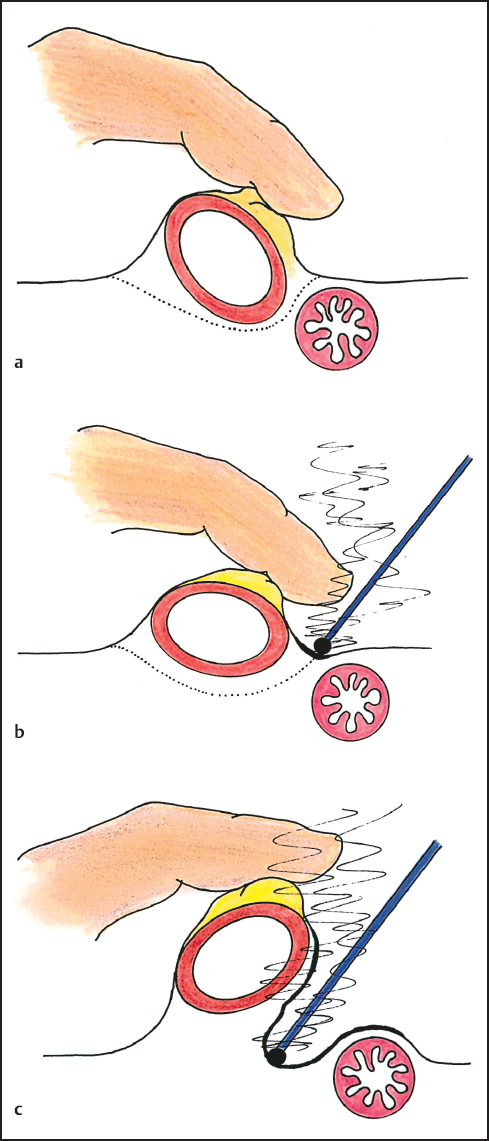
Fig. 13.6 A narrow margin of dissection may be a cancer-free margin if a thin layer of heat necrosis occurs from the electro-evaporative surgery.
 Irrigation and Suction
Irrigation and Suction
Irrigation and suction should be repeated continuously during electrosurgical dissection. Although heat necrosis may provide an essential cancer-free margin of dissection, electrosurgical dissection may provoke heat damage to vital structures, especially tubular structures. Repeated saline irrigation cools dissected tissues and clears tissue debris and blood from the operative field. If blood is repeatedly cleared from within the operative field the translucent nature of the tissues is preserved. This results in minimal inadvertent damage to vital structures. Strong and complete suction is essential to evacuate blood, fibrin, and saline that continuously accumulate within the operative field.
At the end of surgery, copious irrigation of the abdominal cavity with a solution of 1% hydrogen peroxide may help in reducing the number of cancer cells trapped within blood clots. Also, residual blood within the abdomen and pelvis will cause postoperative adhesions. Free cancer cells and tumor emboli that are retained within the operative site may progress rapidly stimulated by growth factors modulating the wound healing process.
The electroevaporation caused by electrosurgery results in copious smoke production. The release of these carbonized tissues into the air creates a health hazard within the operating room environment. Also, smoke obscures clear visualization of the operative field. Especially in deep dissections as in the chest, under the diaphragm, or in the pelvis, smoke will obscure the operative field and makes surgery unsafe. A smoke evacuator is an essential tool necessary for electroevaporative surgery.
 Peritonectomy Procedures
Peritonectomy Procedures
The most fundamental technique of cytoreductive surgery is the peritoneal stripping procedure. With this methodology all of the parietal peritoneum of the abdomen and pelvis involved by tumor is stripped away. The implants on visceral peritoneum are individually electroevaporized if they are small and diffuse. If large tumor nodules are present on small bowel surfaces this portion of the intestine must be resected. The surgeon’s goal must be to remove all visible evidence of cancer implants in order to maximize the benefits of perioperative intraperitoneal chemotherapy. The long-term results of local-regional chemotherapy are optimized if the volume of residual cancer is minimal. The following paragraphs describe the surgical technique of peritonectomy.
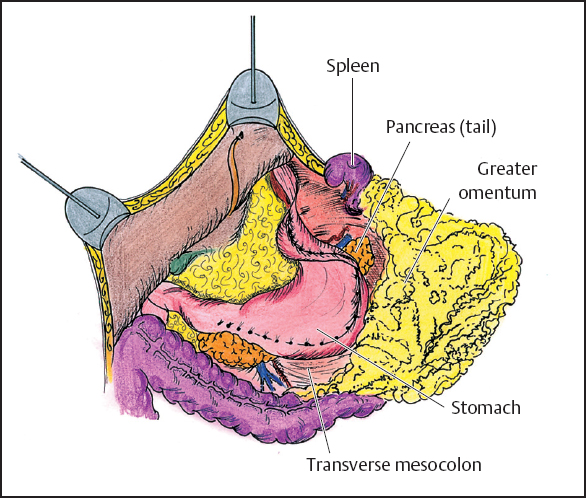
Fig. 13.7 Dissection of the greater omentum.
 Techniques
Techniques
Greater Omentectomy and Splenectomy
The greater omentum is resected in order to free the midabdomen of large volume disease and to improve visualization of the residual abdominal cavity. The greater omentum is elevated and separated from the transverse colon using electroevaporative surgery (Fig. 13.7). The dissection continues beneath the peritoneum that covers the anterior aspect of the transverse mesocolon in order to expose the anterior peritoneal surface of the pancreas. All branches of the gastroepiploic vessels on the greater curvature of the stomach and the short gastric vessels are transected. The last step of this procedure is the resection of the spleen. This allows exposure and complete dissection of tumor implants on the anterior fascia of the pancreas.
Left Upper Quadrant Peritonectomy
In order to strip peritoneum and tumor from the undersurface of the left hemidiaphragm, the dissection of the peritoneum is begun from the posterior sheath of the rectus muscle. The dissected peritoneum is secured and traction maintained with the help of clamps at 10-cm intervals (Fig. 13.8). In performing the left upper quadrant peritonectomy, the following structures are exposed: the undersurface of the left hemidiaphragm muscle, left adrenal gland, distal portion of the pancreas, and cephalad one half of the perirenal fat. The completed left upper quadrant peritonectomy is shown in Figure 13.9.
Right Upper Quadrant Peritonectomy
As in the left upper quadrant peritonectomy, the right upper quadrant peritonectomy begins by stripping peritoneum from the posterior rectus sheath beginning at the edge of the abdominal incision. Dissection is favored by strong traction with clamps and performed with electrosurgery to prevent blood loss (Fig. 13.10). The dissection is continued up to the bare area of the liver. At this point, the eradication of the tumor on the anterior surface of the liver may occur in two different ways. If there are isolated patches of tumor, it is possible to electroevaporize them with the distal 2 cm of the ball-tip bent and freed from insulation (hockey stick configuration). If the tumor involves a major portion of the Glisson’s capsule, the best approach involves a dissection beneath tumor through the capsule itself. If possible, the specimen should be maintained intact. Dissection continues laterally on the right stripping the peritoneum away from the right perirenal fat and thereby freeing the right subhepatic space (Morrison’s pouch) of tumor (Fig. 13.11). In this area the surgeon must be careful not to damage the inferior vena cava and the caudate lobe veins that pass between the vena cava and segment I of the liver. At the end of the procedure, it is possible to see the right upper quadrant freed from peritoneum and tumor between the right costal margin and the liver displaced medially. As shown in Figure 13.12, the following structures are visualized at the completion of the right upper quadrant peritonectomy: the undersurface of the right hemidiaphragm with anterior branch of the phrenic artery and vein, right hepatic vein, inferior vena cava, right adrenal gland, and the cephalad one half of the perirenal fat surrounding the right kidney.
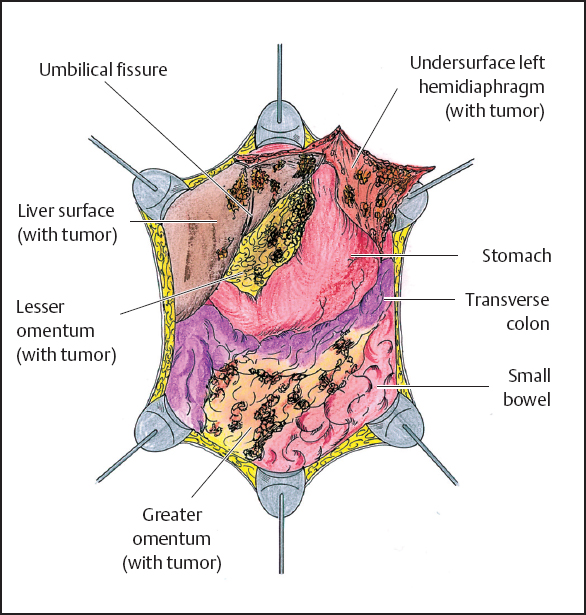
Fig. 13.8 Abdominal cavity after greater omentectomy and splenectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


