Stem Cells in Breast Development and Carcinogenesis: Concepts and Clinical Perspectives
Maria Ouzounova
Suling Liu
Max S. Wicha
There has been accumulating evidence for the existence of a subcomponent of cancer cells that have stem cell properties and have been termed “cancer stem cells.” Although the concept that cancer originates from the transformation of “germ cells” or “stem cells” was first proposed over 150 years ago, it is only recently that advances in stem cell biology have allowed for a more direct testing of the cancer stem cell hypothesis. Stem cells are defined by their ability to undergo self-renewal, as well as multi-lineage differentiation. This self-renewal can be either asymmetric or symmetric. Self-renewal is distinguished from other proliferative processes in that at least one of the progeny of self-renewal is identical to the initial stem cell. In all other replicative processes, the progeny of division undergo a series of differentiation events (1). In asymmetric stem cell self-renewal, one of the two progeny is identical to the initial stem cell, whereas the other cell is a committed progenitor cell, which undergoes cellular differentiation. Because the product of an asymmetric self-renewal division is one stem cell and one differentiated cell, this process maintains stem cell numbers. In contrast, symmetric self-renewal results in the production of two stem cells; by its very nature this results in stem cell expansion. Although stem cells themselves are slowly dividing, progenitor cells derived from them are highly proliferative (2). This expanding progenitor cell also has the ability to differentiate into the lineages comprising the adult tissue. Embryonic stem cells are pluripotent, able to differentiate into all derivatives of the three primary germ layers (ectoderm, endoderm, and mesoderm), whereas adult stem cells are multipotent, able to form all of the cell types that are found in the mature tissue of an organ. In the mammary gland, these differentiating cells generate three lineages: ductal epithelial cells, which line ducts; alveolar epithelial cells, which are the milk-producing cells; and myoepithelial cells, which are contractile cells lining ducts and alveoli.
Based on this definition, cancer stem cells retain key stem cell properties. These properties include self-renewal, which initiates and drives tumorigenesis, and differentiation, albeit aberrant, which contributes to cellular heterogeneity (3)
In breast cancer, the discovery of tumor cells that display stem cell properties provides a possible explanation as to why cancer may be so difficult to eradicate, as well as suggesting strategies for the targeting of this cell population. This chapter will examine the implications of the cancer stem cell hypothesis and enable an understanding of carcinogenesis, as well as its implications for developing new strategies for prevention and therapy of breast cancer.
IDENTIFICATION OF NORMAL BREAST STEM CELLS
The existence of adult mammary stem cells was established nearly 50 years ago when DeOme et al. (4) observed that tissue fragments of epithelium isolated from several different regions of the mammary gland were able to reconstitute the entire mammary ductal tree upon transplantation. Later, serial transplantation experiments by Charles Daniel and colleagues (5) demonstrated that stem cells exist throughout the life span of the mouse. Further studies by Smith and Medina (6) suggested that mammary stem cells were present in all portions of the ductal mammary tree at all developmental stages. In 2006, two complementary studies demonstrated that a single cell from either the CD24lo (heatstable antigen)/CD29hi (α1-integrin) (7) or CD24lo/CD49fhi (α6-integrin) (8) epithelial population isolated from an adult virgin mouse could generate a functional mammary gland when transplanted into the cleared fat pad of recipient mice.
Further analysis of the CD24loCD29hi cells revealed that this was a basal population of cells that was ERα-negative (9). Limiting dilution transplantation experiments by Smalley and co-workers (10) illustrated that CD24lo ERα-negative basal cells displayed the highest stem cell activity (as defined by mammary repopulating units), whereas ERα-positive luminal cells exhibited very little stem cell activity. Conversely, Booth and Smith (11) suggested that long-lived, slow-dividing, label-retaining ERα-positive cells comprise a progenitor cell population that can directly respond to hormones. The relationship of these cells characterized in situ to the CD24lo cells identified by fluorescence activated cell sorting remains to be established. A well-established in vitro system for assays of stem cell behavior—the mammospheres culture system—is a nonadherent assay in which mammary stem cells are cultured as floating cell colonies, without inducing cell differentiation. It was shown that human breast epithelial cells formed mammospheres after 7 to 10 days of culture, which maintained a primitive phenotype and therefore did not express markers associated with terminal differentiation (12, 13). In culture conditions which favored cell differentiation, cells isolated from dissociated mammospheres were shown to have the capacity for multi-lineage differentiation in two dimensional culture (as assessed by expression of cell-type specific markers) and in three-dimensional culture gave rise to lobular-alveolar structures (12).
Ginestier et al. (14) have described the expression of aldehyde dehydrogenase 1 (ALDH1) as a stem cell marker that can be utilized to isolate human mammary stem cells. ALDH1 is a detoxifying enzyme responsible for the oxidation of intracellular aldehydes. This enzyme may play a role in early differentiation of stem cells through its role in oxidizing retinol to retinoic acid (15). It is expressed in hematopoietic and neuronal stem and progenitor cells and can be detected utilizing an enzymatic assay (ALDEFLUOR; Aldagen, Durham, North Carolina) (16). Human mammary epithelial cells with a high enzymatic activity for ALDH (ALDEFLUOR positive), isolated from reduction mammoplasties, were able to reconstitute human mammary gland structures when implanted in the humanized fat pad of NOD/SCID mice. Using ALDH1 antibody to immunostain paraffin-embedded sections of human normal breast epithelium researchers identified a relatively rare population of ALDH1-positive cells located in the terminal ductal lobular units (TDLUs). ALDH1-positive cells appeared to form a bridge in the lumen that was located at the bifurcation point of side branches in the TDLUs (14). This is consistent with recently published data demonstrating that human stem/progenitor cells are localized in the ductal part of the TDLU structures (17).
The identification of mammary stem cell markers and the development of in vitro and murine models utilizing these cells should facilitate the study of adult breast stem cells to elucidate their role in mammary development. Furthermore, defining the pathways that regulate mammary stem cell self-renewal and differentiation should shed light on events involved in breast carcinogenesis.
BREAST CARCINOGENESIS
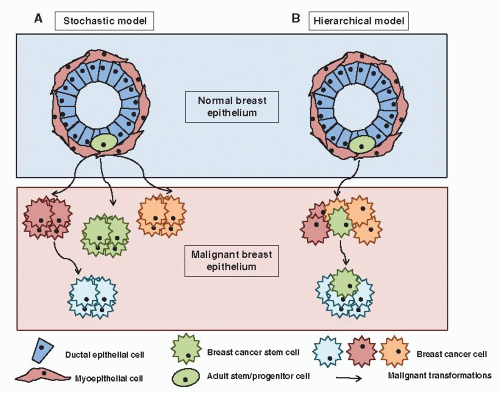
Traditionally, cancer has been considered as a multistep process defined by the sequential mutation of key genes driving the uncontrolled clonal expansion of a cell. However, important recent progress in basic research has challenged these concepts at different levels. First, the role of the tumor microenvironment is now well recognized, including the interaction with the extracellular matrix (ECM) and the immune system (18, 19). Indeed, epithelial cells are dependent on interactions with specific components of the ECM for survival, proliferation, and differentiation. In addition, the initial steps in tumor establishment are associated with a deficiency in the mechanisms of immunosurveillance. Second, the role of epigenetic deregulation, as opposed to genetic aberrations, in most human tumors is becoming increasingly evident. Epigenetic mechanisms appear to play a fundamental role in cancer establishment and progression, and their deregulation has been reported at multiple levels, including DNA methylation, histone modifications, and microRNA expression (20, 21, 22, 23 and 24). Third, a “cancer stem cell” model of tumorigenesis has gained experimental support. This model suggests that tumors are sustained in their pathological growth by a small subpopulation of tumor cells with “stem-like” properties, in a way analogous to normal organogenesis. Cancer stem cell (CSC) is an operational term to functionally define this distinct subpopulation of tumor cells with deregulated potential for self-renewal, excessive proliferation, and aberrant differentiation into heterogeneous progeny, generating intratumor heterogeneity (25, 26) Indeed, classical models of carcinogenesis can be described as “stochastic” or “random,” in which any cell in an organ, such as the breast, can be transformed by the right combination of mutations (27). As a result, all or most of the cells in a fully developed cancer are equally malignant (Fig. 2-1). It follows that strategies designed to treat and ultimately cure these cancers require the killing of all these malignant cells. Conversely, the cancer stem cell hypothesis is a fundamentally different model composed of two separate, but interrelated components. The first is that tumors originate in tissue stem and/or progenitor cells through the deregulation of the normally tightly regulated process of self-renewal (28). As a consequence, it is believed that tumors contain a cellular component that retains key stem cell properties including self-renewal, which initiates and drives carcinogenesis and differentiation, albeit aberrant, that contributes to tumor cellular heterogeneity (Fig. 2-1B) (29). However, it is important to emphasize that the CSC and stochastic model of carcinogenesis are not mutually exclusive and probably both mechanisms contribute to tumor heterogeneity. As a result tumors may be constituted by multiple CSC clones which evolve during tumor development and treatment.
ISOLATION AND CHARACTERIZATION OF BREAST CANCER STEM CELLS
Even though important progress has been made, the isolation and characterization of cancer stem cells remains a challenge. In order to validate the method selected as an appropriate technique to isolate cancer stem cells, it is crucial to use assays that can assess the stem cell properties of self-renewal and differentiation. Presently, the gold standard for identifying breast cancer stem cell activity is the xenograft model based on the orthotopic injection of human breast cancer cells into the humanized clear mammary fat pad of immunodeficient mice. The cancer stem cell population is characterized by enhanced tumorigenicity and is able to regenerate the tumor upon serial passage, whereas the tumor cell population depleted of cancer stem cells cannot sustain tumor growth upon serial transformation (Fig. 2-2). In addition to self-renewal, cancer stem cells retain the ability to differentiate, albeit abnormally, generating non-self-renewing cell populations that constitute the bulk of a tumor. Development
of in vitro assays such as the mammosphere assay has been also used for enrichment of cancer stem cell population. This method is a nonadherent colony forming assay developed by Dontu et al. (30) where only cells with self-renewal capacity are able to survive and grow in anchorage-independent conditions while differentiated cells will undergo anoikis.
of in vitro assays such as the mammosphere assay has been also used for enrichment of cancer stem cell population. This method is a nonadherent colony forming assay developed by Dontu et al. (30) where only cells with self-renewal capacity are able to survive and grow in anchorage-independent conditions while differentiated cells will undergo anoikis.
In summary, several different techniques have been utilized to enrich for and identify breast cancer stem cells. The in vitro cancer stem cell assays provide an important tool for mechanistic studies as well as for screening of specific drugs targeting this population. However, at this time, self-renewal can only be confirmed by serial passage in xenograft models. A potential limitation of these systems relates to the microenvironmental difference found in humans compared to NOD/SCID mice (31). Another important characteristic of both in vivo and in vitro assays to be taken into account is that these techniques may only detect proliferating stem cells but not dormant cancer stem cells.
BCSC MARKERS
The first evidence for the existence of cancer stem cells in human solid tumors came from the study of Al-Hajj et al. (32) where they utilized techniques based on seminal studies identifying leukemic stem cells by Bonnet and Dick (33). Utilizing cell surface markers and flow cytometry, these authors isolated a tumorigenic population of cells in human breast cancer that displayed cancer stem cell properties. This population was defined by the expression of cell surface markers (CD44+/CD24-/low/lin–). When injected in the mammary fat pad of NOD/SCID mice as few as 200 of these cells were able to form tumors, whereas 20,000 cells that did not display this phenotype failed to generate tumors. Tumors that formed in mice recapitulated the phenotypic heterogeneity of the initial tumor. The ability to serially transplant the tumors from an enriched stem cell population provides strong support for the existence of stem cells in breast cancers. CD44 appears to be also expressed in cancer stem cells in other tumor types including colon, pancreas, prostate, and head and neck (34, 35, 36 and 37).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree