Staging and Prognosis of Differentiated Thyroid Cancer
Richard T. Kloos
Introduction
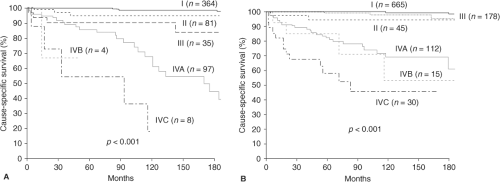
In the United States, the 10-year overall relative survival rates for patients with papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), Hürthle cell thyroid cancer (HTC), medullary, and undifferentiated/anaplastic carcinoma are approximately 93%, 85%, 76%, 75%, and 14%, respectively (1). Together, PTC, FTC, and HTC are known as differentiated thyroid cancer (DTC), and about 30% of such patients have tumor persistence or recurrence (2,3). The ability to immediately predict which patients are at higher risk of tumor persistence, local recurrence, distant recurrence, and tumor-related death is of great interest to patients and care givers, and may influence treatment and follow-up decisions as well as future clinical trial designs (Fig. 50D.1A–B).
This chapter focuses on initial staging and prognostic factors of DTC that are potentially available to the clinician early in the course of the patient’s care. These include initial surgical histopathology and biochemical results, as well as anatomic and/or functional imaging results at the time of a potential post-treatment whole body radioiodine scan. Chapter 50E presents information regarding patient “restaging” over time, which incorporates both the response to previous treatment, and new information that develops over time that may impact both clinical recurrence and disease-specific survival.
Generally Accepted Prognostic Variables
Historically, the most important variables predicting DTC recurrence are direct local tumor invasion (2,3,4,5,6), lymph node metastases (LNMs) (2,3,5,6), and tumor size (2,3,5,6). These factors, plus advanced age, increase the risk of distant metastatic disease (3). For cancer mortality, tumor histology and distant metastases are added (2,3,5,6,7), while the prognostic value of LNM to PTC mortality has long been debated. Prior radiation to the head or neck does not influence the risk of tumor recurrence or death (2,6).
Increasing age has been associated with lower relative survival for DTC, medullary, and anaplastic thyroid carcinomas (1,8). In DTC series (where PTC cases are the majority), age is notoriously a challenging variable, as youth and older age are both associated with local and distant disease recurrence, while only older age is associated with an increased risk of cancer-related death. In contrast, young patients have a much higher survival rate, even in the setting of distant metastases (3,6,9,10,11). In addition, whereas localized DTC has an excellent prognosis and weak dependence on age, those with regional or distant metastases demonstrate a survival rate this is more strongly dependent on age (11,12). Most staging systems (Table 50D.1) include age as a variable, although often only as a bimodal variable. This limitation is demonstrated by the American Joint Committee on Cancer (AJCC) Tumor–Node–Metastasis (TNM) Staging System (Table 50D.2) where a 44-year-old T4bN1M0 patient is stage I, but the same tumor at age 45 is stage IVB (13).
Table 50D.1 Components of Staging Systems to Define Risk Category in Differentiated Thyroid Carcinoma (Dtc) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinical Staging Systems for Disease Recurrence and Patient Survival
Multiple staging systems have been described (6,7,9,14,15,16,17,18,19,20,21), the most commonly employed of which are shown in Table 50D.1. The advantages of staging systems include the simplification of multiple variables from one patient into a single “stage,” the ability to communicate and categorize this information for clinical care decisions, and to permit comparisons of outcome among research studies. However, these staging systems imperfectly predict the outcome of each individual patient, and they fail to incorporate all the known prognostic variables; for example, the DTC tumor subtype and fluorodeoxyglucose positron emission tomography (FDG-PET) avidity.
Table 50D.2 Scoring Method for Tumor-Node-Metastasis (Tnm) Seventh Edition (13) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
The TNM Staging System was designed to predict cause-specific survival, not recurrence rates. It is currently in its seventh edition (13). Lang et al. (22) evaluated the changes in cause-specific survival (CSS) from a cohort of 760 DTC patients after revision from the fifth edition published in 1997 (23) to the sixth (9) edition published in 2002. In the sixth edition, T1 tumors change from ≤1 cm confined to the thyroid to ≤2 cm tumors confined to the thyroid, T2 tumors changed from >1 to 4 cm to >2 to 4 cm, T3 tumors changed from tumors >4 cm in greatest dimension limited to the thyroid to also include any tumor with minimal extrathyroidal extension (e.g., extension to sternothyroid muscle or perithyroidal soft tissues), and T4 tumors that previously included tumors extending beyond the thyroid capsule became reclassified to T3 or T4a if there was extrathyroidal extension into subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve; or T4b if tumor invaded the pre-vertebral fascia or excased the carotid artery or mediastinal vessels. In the sixth edition, N1a LNMs changed from metastases in the ipsilateral cervical lymph nodes to level IV LNMs and N1b changed from
bilateral, midline, or contralateral cervical or mediastinal lymph nodes to unilateral, bilateral, or contralateral cervical or superior mediastinal lymph nodes. Regarding stages, there was no change for patients under age 45 years. At the age of 45 years and over, stage I remained T1N0M0 (recognizing the new definition of T1). Stage II again included T2N0M0 (recognizing the new definition of T2), but T3N0M0 patients moved from the fifth edition stage II to the sixth edition stage III. Stage III changed from T4N0M0 or Any TN1M0 to include T3N0M0 and T1-3N1aM0. Finally stage IV changed from M1 patients to stage IVA (T4aN0-1bM0, T1-3N1bM0), IVB (T4bAny NM0), and IVC (Any T Any NM1). Upon reclassification, the proportion of T1 and T3 tumors increased, N1a decreased, N1b increased, and stages I and IV tumors increased. The sixth edition better predicted CSS among the stages. Wada et al. (24) also evaluated the prognostic value of the fifth (23) and sixth editions (9) and found no advantages in the new definitions of T1 and T2 or N category, but concluded that the sixth edition more accurately predicted outcome in patients with extrathyroidal extension. The excellent survival outcome of nearly all stage I patients was demonstrated. These important findings have also been demonstrated by others (25) (Fig. 50D.1A–B). In another study, Wada et al. (26) reported that within sixth edition TNM stage IVA PTCs, those with
T4aN1b disease have significantly impaired disease-free survival (DFS) and disease-specific mortality compared to other stage IVA patients. The current seventh edition (13) (Table 50D.2) further subdivides T1 primary tumors into T1a and T1b categories with no published reports available on the contribution of this subdivision to predict DFS.
bilateral, midline, or contralateral cervical or mediastinal lymph nodes to unilateral, bilateral, or contralateral cervical or superior mediastinal lymph nodes. Regarding stages, there was no change for patients under age 45 years. At the age of 45 years and over, stage I remained T1N0M0 (recognizing the new definition of T1). Stage II again included T2N0M0 (recognizing the new definition of T2), but T3N0M0 patients moved from the fifth edition stage II to the sixth edition stage III. Stage III changed from T4N0M0 or Any TN1M0 to include T3N0M0 and T1-3N1aM0. Finally stage IV changed from M1 patients to stage IVA (T4aN0-1bM0, T1-3N1bM0), IVB (T4bAny NM0), and IVC (Any T Any NM1). Upon reclassification, the proportion of T1 and T3 tumors increased, N1a decreased, N1b increased, and stages I and IV tumors increased. The sixth edition better predicted CSS among the stages. Wada et al. (24) also evaluated the prognostic value of the fifth (23) and sixth editions (9) and found no advantages in the new definitions of T1 and T2 or N category, but concluded that the sixth edition more accurately predicted outcome in patients with extrathyroidal extension. The excellent survival outcome of nearly all stage I patients was demonstrated. These important findings have also been demonstrated by others (25) (Fig. 50D.1A–B). In another study, Wada et al. (26) reported that within sixth edition TNM stage IVA PTCs, those with
T4aN1b disease have significantly impaired disease-free survival (DFS) and disease-specific mortality compared to other stage IVA patients. The current seventh edition (13) (Table 50D.2) further subdivides T1 primary tumors into T1a and T1b categories with no published reports available on the contribution of this subdivision to predict DFS.
The many staging systems available include a number of prognostic variables, but no system includes all of them. Systems developed on the basis of cohorts of DTC typically reflect the distribution of histologic subtypes seen in clinical settings, and are dominated by PTC, and thus may not be applicable for less common subtypes. Further, the variance of patient outcome is only partially predicted by these staging systems (27,28,29) which may reflect their deficiencies (e.g., variables not included or appropriately weighted), their general lack of incorporating treatment variables (e.g., extent of surgery) (30), inability to predict future changes in any residual tumor (e.g., tumor dedifferentiation), or lack of including response to treatment (“restaging”). The possibilities of these shortcomings are seen in the results of studies that demonstrate beneficial effects of surgery, extent of surgery, thyroid stimulating hormone (TSH) suppression, or radioiodine remnant ablation and/or adjuvant use, or treatment of metastases.
Combined Series of Differentiated Thyroid Cancer
Brierley et al. (27) performed a retrospective review of 382 patients with PTC and FTC regarding CSS and found that age–grade–extent–size (AGES), fourth edition TNM (31), age–metastasis–extent–size (AMES), European Organization for Research and Treatment of Cancer (EORTC) (Table 50D.3), and Metastasis–Age–Completeness of resection–Invasion–Size (MACIS) (Table 50D.4) staging systems performed similarly, and that they outperformed the OSU, Memorial Sloan–Kettering (MSK), and Clinical Class staging systems.
Table 50D.3 Scoring Method for Eortc (16) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Table 50D.4 Scoring Method for Macis (Metastasis-Age-Completeness of Resection-Invasion-Size) (7) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Passler et al. (28) assessed 440 patients with DTC to determine the predictive accuracy of nine staging systems [EORTC, fifth edition TNM (23), MACIS, AMES, Clinical Class, OSU, MSK, University of Alabama at Birmingham/M.D. Anderson Cancer Center (UA & MDA), and University of Münster], toward cause-specific mortality (CSM) and found that MACIS was best, followed by the EORTC and fifth edition TNM.
In a multivariate analysis for DFS and overall survival (OS) in 382 patients, Gulcelik and colleagues (32) reported that distant metastasis, FTC, tumor extension beyond the thyroid capsule, vascular invasion (not included in most staging systems), primary tumor size, sixth edition TNM stage, and high MACIS score were independent prognostic factors, while the AMES score was not.
Taken together, these studies indicate that among staging systems, MACIS, EORTC, and TNM staging systems have the strongest utility to predict DTC-related death.
Papillary Thyroid Cancer
Brierley et al. (27) performed a retrospective review of PTC regarding CSS and found that AGES, fourth edition TNM, AMES, EORTC, and MACIS performed similarly, and that they were superior to OSU, MSK, and Clinical Class.
Voutilainen et al. (29) compared the AMES, MACIS, and the fifth edition TNM staging systems toward predicting carcinoma-specific mortality in 495 PTC patients. Carcinoma-specific mortality in the low-risk group of AMES, MACIS, and TNM were 2.4%, 2.4%, and 1.2%, respectively. The mortality ratio at 10 years between high-risk and low-risk patients was 22.2 for AMES, 25.0 for MACIS, and 41.8 for TNM. The proportion of explained variance in the Cox model was 16.3 for AMES, 30.0 for MACIS, and 28.9 for TNM. The authors concluded that TNM was superior in predicting PTC cause-specific mortality.
Passler et al. (28) assessed 440 patients with DTC to determine the predictive accuracy of nine staging systems [EORTC, fifth edition TNM (23), MACIS, AMES, Clinical Class, OSU, MSK, UA & MDA, and University of Münster] for CSM and found MACIS superior for PTC.
Lang et al. applied 14 systems to 589 PTC patients. All 14 staging systems significantly predicted CSS. The three highest ranked staging systems were MACIS, followed by sixth edition TNM, and EORTC (19). In a subsequent study, nine systems were applicable to 1,634 PTC patients within two tertiary referral centers (Table 50D.5, Fig. 50D.1A–B). The authors reported that despite referral, treatment, and data collection biases inherent within each center, the sixth edition TNM system was the most applicable and consistent staging system (25). Still, MACIS, sixth edition TNM, and EORTC similarly outperformed the other systems.
Taken together, these studies indicate that among staging systems, MACIS, EORTC, and TNM staging systems have the strongest utility to predict PTC-related death.
Follicular Thyroid Cancer
Davis et al. (33) compared EORTC, AGES, and AMES scores in 122 patients for survival and found that the AGES and EORTC scoring systems best defined low- and high-risk groups of patients with pure FTC, although the separation between the groups was low.
Passler et al. (28) assessed 440 patients with DTC to determine the predictive accuracy of nine staging systems and found that EORTC and fifth edition TNM (23) best predicted CSM in FTC.
D’Avanzo et al. (34) studied 86 patients with FTC and reported that fifth edition TNM, EORTC, AGES, AMES, and MACIS all provided useful prognostic information regarding survival with FTC. The MACIS classification, however, was the most accurate predictor.
Lo et al. (35) studied 156 FTC patients after histologic reclassification according to the type and degree of tumor invasiveness. Older age, distant metastases, and incomplete tumor excision were independent prognostic factors for survival. AMES, fifth edition TNM, Clinical Class, and MACIS scoring schemes all predicted CSS, although TNM yielded the best information.
Lang et al. studied 14 different staging systems to predict CSS in 171 FTC patients. The three highest ranked staging systems according to the proportion of variation in survival time explained were sixth edition TNM, followed by Clinical Class, and MACIS (36).
Taken together, these studies indicate that amongst staging systems, MACIS, EORTC, and TNM have the strongest utility to predict FTC-related death.
Differentiated Thyroid Cancer Subgroups and Variants
Multiple studies have demonstrated outcome differences among the various tumor histologies, although most DTC staging systems do not differentiate between PTC and FTC, let alone their variants. Among 53,856 thyroid cancer cases, the 10-year overall relative survival rates for U.S. patients with PTC, FTC, and HTC are approximately 93%, 85%, and 76%, respectively (1). The World Health Organization (WHO) does not recognize an independent HTC tumor group, but includes it as a variant of PTC or FTC groupings (37). WHO recognizes histologic variants of the major carcinoma groups when the tumor is dominated by a special feature such as its growth pattern, cell type, or stromal reaction. In general, the PTC histologic variants with better prognoses include the follicular variant, macrofollicular variant, oncocytic variant, clear cell variant, cribriform carcinoma, papillary carcinoma with fasciitis-like stroma, combined papillary and medullary carcinoma, and papillary microcarcinoma (PMC) (37). Conversely, the PTC variants with worse prognoses include the diffuse sclerosing variant (DSV), tall cell variant, columnar cell variant, solid variant, papillary carcinoma with squamous cell or mucoepidermoid carcinoma, papillary carcinoma with focal insular component (IC), and papillary carcinoma with spindle and giant cell carcinoma. FTC variants include the oncocytic and clear cell variants (37). Prognostic information regarding these histologies and variants is selectively reviewed below. In addition, tumor differentiation status (e.g., tumor necrosis, numerous mitoses, or marked nuclear atypia) for a given histologic type independently impacts prognosis (8,38).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree