Summary of Key Points
- •
Stereotactic ablative radiotherapy (SABR) is recommended in treatment guidelines as the nonoperative therapy of choice for early-stage nonsmall cell lung cancer (NSCLC).
- •
SABR can be adequately performed using either traditional linear accelerators equipped with suitable image-guidance technology or linear accelerators specifically adapted for SABR and using dedicated delivery systems.
- •
Clinical assessment, staging of disease, and multidisciplinary discussion should be based on published guidelines for early-stage NSCLC.
- •
Guideline-specified nodal staging should be performed before SABR, as nodal regions are not radiated.
- •
SABR dose constraints, which are based on the constraints used in the RTOG 0618, 0813, and 0915 SABR trials, are summarized in the current National Comprehensive Cancer Network guidelines.
- •
Results of SABR have been consistent, with both the high local control rates and low toxicity found in prospective clinical trials also being reported in large single-institution series and pooled multi-institutional analyses.
- •
A widely used working definition for so-called central lung tumors is tumors located either adjacent to the proximal bronchial tree or located 1 cm or less from the heart or mediastinum.
- •
Three distinct cohorts of patients with oligometastases can be identified: patients with oligometastatic disease at the time of diagnosis, patients with oligoprogressive disease after cytoreductive therapy, and patients with oligorecurrent disease after curative local–regional therapy.
- •
Results appear generalizable across centers when current SABR guidelines are followed.
Changes in the epidemiology of lung cancer are particularly relevant for the field of radiation oncology. Globally, lung cancer represents the leading cause of cancer death in men and is the second leading cause in women. A key challenge is that older patients are the fastest growing population—nearly 25% of patients are 75 years of age or older. Approximately 20% of all patients diagnosed with nonsmall cell lung cancer (NSCLC) have stage I disease, and surgery is currently the guideline-specified treatment for fit patients who are willing to accept the procedure-related risks. However, a population study from the Netherlands showed that, among patients with stage I disease, resection was done in 49% of patients 75 years of age or older compared with 91% of patients who were 60 years of age or younger. Similarly, in an analysis of data in the Surveillance, Epidemiology and End Results (SEER)-Medicare database for the period 1998 to 2007, the percentage of patients who had a surgical procedure decreased over time (75.2% in 1998 vs. 67.3% in 2007) and the percentage of patients who did not receive any local treatment increased (14.6% in 1998 vs. 18.3% in 2007). These findings were explained by the increase in the proportion of patients 85 years of age or older (from 4.5% to 9%), as well as an increase in patients with three or more comorbidities (from 15% to 30%) during the study period. The reluctance to operate on older patients is mainly due to their frailty, as comorbidities are more common in the older population. Although severe comorbidity has the greatest impact on outcomes during the first month following surgery, the increased death rate associated with impaired performance status persists with longer follow-up.
The apparent reluctance of clinicians to refer older patients for conventional radiotherapy was partly due to the 30 or more once-daily treatments that were typically required, which is cumbersome for frail older patients. In the era before stereotactic ablative radiotherapy (SABR), outcomes of radiotherapy in early-stage NSCLC were poor despite treatment with doses ranging from 60 to 66 Gy. Local tumor recurrences occurred in approximately 40% of patients, with an overall survival rate at 3 years of approximately 30%. Furthermore, a modest 6-month increase in median survival was reported in an analysis of 2010 SEER data when older radiotherapy techniques were applied.
SABR: Background and Definitions
In the mid-1990s, the principles of cranial stereotactic radiotherapy (or radiosurgery) were transferred to extracranial sites by work pioneered at the Karolinska Hospital in Sweden. Stereotactic body radiation therapy (SBRT) and SABR are equivalent terms for this technique in the body. This stereotactic approach was further developed by centers in Japan and Germany. In subsequent years, encouraging results from both prospective and retrospective studies resulted in rapid adoption of SABR for early-stage NSCLC. A national survey in the United States found that 57% of all responding physicians used SABR for the treatment of lung cancer in 2010, whereas a similar survey in Italy found that 41% of responding radiotherapy centers used SABR in 2009. At present, SABR is recommended in treatment guidelines as the nonoperative therapy of choice for early-stage NSCLC.
Guidelines for SABR have been released by several professional groups: the American Association of Physics in Medicine Task Group 101, the American Society for Therapeutic Radiology and Oncology and the American College of Radiology, the Canadian Association of Radiation Oncology-Stereotactic Body Radiotherapy, the National Radiotherapy Implementation Group of the UK, and the working group Stereotactic Radiotherapy of the Germany Society of Radiation Oncology. Current definitions of SABR adhere to the following criteria: a high degree of accuracy, use of high doses of radiation, and delivery of radiation in one or a few treatment fractions to an extracranial target.
The rationale of SABR for early-stage NSCLC is that higher radiation doses are more effective for local control of the tumor, which translates into longer overall survival. SABR differs from conventional radiotherapy in that SABR involves delivery of very high radiation doses only to the visible tumor, with planning and delivery of treatment optimized to ensure safety margins of a few millimeters. In addition, radiation doses to surrounding normal organs are often lower than with conventional techniques. As a result, local tumor control rates of 90% and higher can be achieved and rates of severe toxicity are typically below 10%.
SABR for lung cancer is a multidisciplinary endeavor, involving all disciplines related to the diagnosis and treatment of the disease, but particularly specialists working on a radiotherapy team. Accuracy of delivery is achieved using an optimized workflow and appropriate quality assurance procedures, including development of written protocols, which is an essential component of the process.
SABR can be adequately performed using either traditional linear accelerators equipped with suitable image-guidance technology or linear accelerators specifically adapted for SABR and using dedicated delivery systems. The SABR procedure was initially defined by the use of frame-based patient set-up, the goal of which was stable and reproducible patient positioning. However, frame-based stereotactic patient set-up has been replaced by image guidance, which makes the term stereotactic somewhat misleading. With non–frame-based patient set-up, external stereotactic coordinates are replaced by visualization of a patient’s anatomy using images acquired on-table and subsequently compared with pretreatment planning images. Soft-tissue images of the tumor itself, or of an implanted fiducial marker, can be used for setting up the target ( Fig. 37.1 ).
SABR Protocol Development, Implementation, and Quality Assurance
A dedicated SABR team should consist of radiation oncologists, medical physicists, and technicians (e.g., radiographers, radiation therapists), all of whom should have attended appropriate training courses organized by professional bodies and/or industry in accordance with the above-mentioned guidelines. Written treatment protocols that are consistent with national regulations, institution-specific equipment, and training and education of the individual radiotherapy team members should be available. SABR requires additional and more frequent physical quality assurance: verification and quality assurance of the entire SABR treatment chain is mandatory, and end-to-end tests for overall uncertainty estimation are recommended. It is paramount to verify that the radiation isocenter coincides with the mechanical isocenter, including couch rotation, room lasers, and, especially, the imaging isocenter.
Clinical Assessment
Clinical assessment, staging of disease, and multidisciplinary discussion should be based on published guidelines for early-stage NSCLC. Unlike the toxicity found following surgery in older individuals, no increased toxicity or treatment-related mortality has been noted when SABR has been extensively applied to patients between 75 and 80 years old. The poorer overall survival rates reported in this older population after SABR are related to comorbidities, and the number of comorbidities predicts overall survival after both SABR and surgery. SABR-related toxicity is also not increased in patients with very poor pretreatment pulmonary function, and the available data suggest that SABR should be offered to all patients regardless of age and preexisting pulmonary comorbidities, unless their predicted survival time is short.
Diagnosis and Staging Before SABR
Diagnosis and confirmation of NSCLC based on tissue biopsy is recommended before starting any local treatment for early-stage NSCLC. However, obtaining a histologic diagnosis may not be possible for peripheral lung lesions or may pose a high risk of toxicity in a patient group with considerable medical and/or pulmonary comorbidities. In the latter case, radiographic criteria of malignancy are used to establish a diagnosis. Models that predict the probability of malignancy in solitary pulmonary nodules based on both clinical and radiographic characteristics have been described and validated. It is important to note that these criteria may not hold true in geographic regions with a high incidence of infectious and/or granulomatous lung diseases. Therefore, current guidelines state that any treatment for a possible early-stage NSCLC without a pathologic diagnosis should proceed only after assessment by an experienced multidisciplinary tumor board. If the clinical and radiographic findings are inconclusive, repeated imaging to evaluate the growth pattern is an option for some patients, but careful follow-up is required because patients with malignancy are at risk for early disease progression.
Guideline-specified nodal staging should be performed before SABR, as nodal regions are not radiated. Staging with 18 F-2-deoxy- d -glucose-positron emission tomography (FDG-PET) is essential because of its higher diagnostic accuracy for the detection of node metastases (negative predictive value, 90%) and also because unsuspected distant metastases and second primary tumors can be excluded. In the event of pathologic FDG uptake in regional lymph nodes, further evaluation by endobronchial ultrasound or endoscopic ultrasound is recommended and, if the findings are inconclusive, a mediastinoscopy may be necessary. Staging with PET–computed tomography (CT) should ideally be performed within 6 to 8 weeks before SABR is given because of the risk of disease progression in the interim.
Technical Overview for Radiation Oncologists
Target Volume Definition and Treatment Planning
All imaging should be acquired in the treatment position, with standard practice dictating that planning CT images encompass the entire lung volume, and a slice thickness of 2 mm to 3 mm is used. Using intravenous contrast medium may improve the delineation of centrally located primary tumors. As conventional three-dimensional (3-D) CT risk introduces artifacts and systematic errors, four-dimensional (4-D) CT, also known as respiration-correlated CT, is the recommended technique for SABR planning. Although a single 4-D CT planning image provides only a snapshot of a patient’s breathing pattern, several studies have demonstrated that the motion pattern and amplitude are stable over time, making routine repeated 4-D CT imaging unnecessary. The use of FDG-PET alone is not appropriate for a reliable assessment of target motion in SABR planning.
Target Volume Concept and Motion Management Strategy
Gross tumor volume is determined on the basis of CT findings in the lung and soft-tissue window. Current guidelines do not recommend the use of clinical target volume margins when SABR is delivered, as the high radiation doses combined with rather flat dose profiles in pulmonary tissue of low electron density result in sufficient coverage of potential microscopic disease extension. Integration of breathing-induced target motion into the target volume concept will ensure patient-tailored tumor targeting, with clinical implementation dependent on the chosen motion management strategy. Several different approaches are used in routine clinical practice. Continuous radiation in free breathing is performed using the internal target volume concept, the mean target position concept, or real-time tumor tracking. Tumor tracking is possible using dedicated robotic delivery machines, by dynamic multileaf tracking, a gimbaled multileaf collimator, or a dynamic treatment couch. Noncontinuous radiation of the tumor in a reproducible position is performed using gated beam delivery in predefined phases of the breathing cycle, in voluntary breath-hold, or in breath-hold using the active breathing coordinator.
It is important to emphasize that active motion management strategies, such as gating and tracking, require continuous intrafractional monitoring; however, continuous intrafractional monitoring is less critical for passive strategies, such as the internal target volume or mean tumor position concept. Although patient-specific motion management is strongly recommended, data from the available prospective trials did not involve the use of advanced motion management strategies. Of the individualized 4-D motion management strategies used, resulting field sizes are largest for the internal target volume concept; however, this motion management strategy is straightforward to implement and ensures adequate target coverage. Even if all uncertainties in treatment planning and delivery are minimized by currently available technologies, residual errors still remain and require minimum planning target volume (PTV) margins of about 5 mm.
Dose Fractionation and Prescription
Dose prescription and reporting should comply with the International Committee on Radiation Units and Measurements Report on Prescribing, Recording, and Reporting Intensity-Modulated Photon-Beam Therapy as closely as possible, but historical practice and experiences need to be considered as well. Most prospective and retrospective studies used inhomogeneous dose distributions within the PTV, with maximum doses ranging between 105% and 150% of the prescribed dose. Inhomogeneous dose distributions offer the opportunity to deliver an extra dose to the center of the PTV, where the (potentially hypoxic) macroscopic tumor is located, without increased doses to the peripheral normal tissue.
As a result of large differences in single-fraction and total doses between SABR studies, a comparison of physical doses is less meaningful. The linear quadratic model has been widely used for modeling of SABR outcomes data, but it has not been validated for very high single doses. Despite uncertainty surrounding the linear quadratic model, several groups have independently demonstrated a clear dose-effect relationship for local tumor control using biologic effective doses (BEDs), with a minimum PTV dose of more than 100 Gy BED (α/β ratio, 10 Gy) required for local tumor control rates of higher than 90%. The current recommended tumor dose for SABR of lung tumors is a minimum of 100 Gy BED, prescribed to the target volume encompassing isodose. A meta-analysis demonstrated a potential detrimental effect of SABR doses exceeding more than 146 Gy BED.
Total doses are typically delivered in one to eight fractions, but insurance reimbursement rules have resulted in a widespread use of five or fewer fractions in the United States. However, use of very high single-fraction doses and total doses (e.g., delivery of three fractions of 20 Gy) can damage normal tissues in or adjacent to the target volume. Consequently, treatment of tumors in proximity to critical normal organs has led to the use of so-called risk-adapted fractionation schemes that deliver the required dose of 100 Gy BED in a larger number of treatment fractions with lower single-fraction doses. Fractionation appears to spare some critical normal organs while ensuring sufficiently high doses to achieve local tumor control.
Fractionation appears to be especially valuable in SABR for centrally located tumors, as it allows for radiobiologic sparing of critical organs such as large bronchi, vessels, heart, and esophagus. High-quality prospective data on the safety and efficacy of SABR for centrally located lesions are limited, but a systematic review of the literature demonstrated local control rates of 85% or greater when the prescribed BED to the tumor was 100 Gy or higher. The overall treatment-related mortality was 2.7%, and when the BED to normal tissue was 210 Gy or lower (α/β ratio, 3 Gy), the rate was 1.0%. Until mature prospective multicenter data become available, a recommended fractionation scheme for experienced centers is 8 × 7.5 Gy, with D max (PTV) of 125%.
Treatment Planning
The voxel size of the dose calculation grid should be 2 mm or less, and both heterogeneity correction and use of type B algorithms improve accurate dose calculation, especially at the interface of lung tissue and soft tissue. Monte Carlo dose-calculation algorithms achieve the most accurate results, but differences to collapsed cone algorithms appear small. All published prospective trials have used 3-D conformal treatment planning. Intensity-modulated radiation therapy and advanced rotational techniques such as volumetric modulated arc therapy have the potential to increase dose conformity and homogeneity and reduce treatment delivery times. More data are needed on the biologic consequences of potential interplay effects between multileaf collimator motion and tumor motion. The effects of using flattening filter-free radiation, in particular, for faster delivery remain unclear. Single-institution data suggest that the flattening filter-free technique is both safe and effective. Published tolerance doses for SABR have largely not been validated, although adherence to published protocols appears reasonable at this time, as rates of severe toxicity were low as a result ( Table 37.1 ). These dose constraints, which are based on the constraints used in the RTOG 0618, 0813, and 0915 SABR trials, are summarized in the current National Comprehensive Cancer Network guidelines.
| Organ at Risk | One Fraction (RTOG 0915) | Three Fractions (RTOG 0618/1021) | Four Fractions (RTOG 0915) | Five Fractions (RTOG 0813) | Eight Fractions |
|---|---|---|---|---|---|
| Trachea and large bronchus | D max 20.2 Gy | D max 30 Gy | D max 34.8 Gy 15.6 Gy < 4 cc | D max 105% b 18 Gy < 5cc c | D max 44 Gy |
| Heart | D max 22 Gy 16 Gy < 15 cc | D max 30 Gy | D max 34 Gy 28 Gy < 15 cc | D max 105% b 32 Gy < 15 cc | — |
| Esophagus | D max 15.4 Gy 11.9 Gy < 5 cc | D max 25.2 Gy 17.7 Gy < 5 cc | D max 30 Gy 18.8 Gy < 5 cc | D max 105% b 27.5 Gy < 5 cc c | D max 40 Gy |
| Brachial plexus | D max 17.5 Gy 14 Gy < 3 cc | D max 24 Gy 20.4 Gy < 3 cc | D max 27.2 Gy 23.6 Gy < 3 cc | D max 32 Gy 30 Gy < 3 cc | D max 36 Gy |
| Chest wall | D max 30 Gy 22 Gy < 1 cc | 30 Gy < 30 cc 60 Gy < 3 cc | D max 27.2 Gy 32 Gy < 1 cc | 30 Gy < 30 cc 60 Gy < 3 cc | — |
| Spinal cord | D max 14 Gy 10 Gy < 0.35 cc | D max 18 Gy | D max 26 Gy 20.8 Gy < 0.35 cc | D max 30 Gy 22.5 Gy < 0.25 cc | D max 28 Gy |
a Radiation Therapy Oncology Group (RTOG) protocols can be found on the RTOG website at www.rtog.org/ClinicalTrials/ProtocolTable.aspx .
b Planning target volume prescription.
Patient Immobilization and Set-Up
Customized patient immobilization devices such as the stereotactic body frame or vacuum cushions have been used, but are not considered essential; although they may improve intrafractional patient stability, they do not negate the need for image guidance. A number of studies demonstrated that image-based verification of the target position has the single largest effect on improving the accuracy of lung SABR. Average internal shifts of 5 mm to 7 mm of the pulmonary target relative to the osseous structure are regularly found, and this shift may exceed 2 cm in individual patients. Therefore, daily pretreatment imaging is performed with online correction of set-up errors and baseline shifts—imaging needs to demonstrate the lung tumor directly, or implanted markers as a surrogate, for the tumor position (see Fig. 37.1 ). Imaging during or after completion of SABR serves as a quality assurance purpose, especially in single-fraction SABR. Several technologies for image guidance are commercially available, and superiority of one method over the other has not been demonstrated. Use of volumetric imaging, as opposed to only implanted fiducial markers, has the advantage of allowing for assessment of changes in target shape and position relative to the position of organs at risk.
Clinical Results of SABR
The strongest evidence in support of SABR for treating early-stage NSCLC comes from population-based data. In one population-based study from the Netherlands, survival rates for patients 75 years of age and older were improved after widespread access to SABR. This finding was attributed to both a reduction in the proportion of untreated patients after short-course SABR became available, as well as to local control rates of up to 90% found with this modality. Of the nearly 30% of older Dutch patients who remained untreated despite the availability of SABR, the 30- and 90-day mortality rates measured from the date of diagnosis for untreated patients were 17.9% and 33.3%, respectively. The 90-day mortality rate may be a result of extensive comorbidity with competing causes of death in this patient population. Therefore, overtreatment in very frail patients, even with SABR, may be avoided by carefully reassessing some less-fit patients a few weeks after diagnosis. Another population-based analysis of the National Cancer Data Base (NCDB) demonstrated improved survival in elderly (aged ≥70 years) patients with early-stage medically inoperable NSCLC receiving SABR compared with observation alone. In an analysis of SEER data, survival rates following SABR were similar to those after lobectomy, with poorer outcomes following conventional radiotherapy or observation only.
The results of SABR have been very consistent, with both the high local control rates and low toxicity found in prospective clinical trials also being reported in large single-institution series and pooled multi-institutional analyses ( Table 37.2 ). Data from these sources demonstrate an average 90% rate of freedom from local progression at 2 years to 3 years. The timing of disease recurrence following SABR was reported in a series of 676 patients, all of whom were treated with a BED of more than 100 Gy ( Table 37.3 ). At a median follow-up of 33 months, median overall survival was 40.7 months; actuarial 2-year rates of local, regional, and distant recurrence were 4.9%, 7.8% and 14.7%; and actuarial 5-year rates were 10.5%, 12.7%, and 19.9%, respectively.
| Author (Year) | No. of Patients | Patients With Histopathologic Confirmation of NSCLC (%) | Overall Survival at 2–3 Years (%) | Freedom From Local Progression at 2–3 Years (%) |
|---|---|---|---|---|
| Prospective Phase II Trials | ||||
| Nagata et al. (2005) | 45 | 100 | 75 | 98 |
| Baumann et al. (2009) | 57 | 67 | 60 | 92 |
| Fakiris et al. (2009) | 70 | 100 | 43 | 88 |
| Ricardi et al. (2010) | 62 | 65 | 51 | 88 |
| Bral et al. (2010) | 40 | 100 | 52 | 84 |
| Timmerman et al. (2010) | 54 | 100 | 38 | 98 |
| All prospective studies a | 328 | 87.6 | 52.1 | 91.2 |
| Large Retrospective Series | ||||
| Grills et al. (2010) | 434 | 64 | 60 | 94 |
| Senthi et al. (2012) | 676 | 35 | 55 | 95 |
| Guckenberger et al. (2013) | 514 | 85 | 46 62 b | 80 93 b |
| All retrospective studies a | 1624 | 58.8 | 53.5 | 90.0 |
a The weighted average values are calculated for the summary of all prospective and retrospective studies.
b Subgroup of 164 patients treated with ≥106 Gy biologic effective dose.
| Event | Median Time to Event (Mo) |
|---|---|
| Recurrence | |
| Local | 14.9 (95% CI, 11.4–18.4) |
| Regional | 13.1 (95% CI, 7.9–18.3) |
| Distant | 9.6 (95% CI, 6.8–12.4) |
| Second primary tumors | 18 (95% CI, 12.5–23.5) |
Three randomized trials were initiated to evaluate SABR versus surgery for early-stage NSCLC. All three closed early due to poor accrual. A pooled analysis of two of the trials was reported. The rate of 3-year overall survival was 79% in the surgery group and 95% in the SABR group ( p = 0.037). The rate of recurrence-free survival at 3 years was similar in the SABR and surgery groups (86% vs. 80%, respectively; p = 0.54). Since there were only 58 patients in the pooled analysis, it is difficult to make conclusions on the superiority of either treatment. However, it does confirm the efficacy of SABR in the absence of patient selection bias that is inherent in phase I and II trials and the use of SABR as an alternative to surgery.
Patterns of early disease recurrence after curative SABR in early-stage NSCLC are similar to recurrences after primary surgery, where the predominant pattern of disease recurrence is also one of distant recurrence, despite staging FDG-PET. This pattern suggests that occult distant metastases at the time of initial diagnosis remain a major challenge. In an analysis of nearly 1300 patients who had resection, the risk of subsequent disease recurrence ranged from 6% to 10% per person-year during the first 4 years after surgery, but decreased thereafter to 2%. Conversely, the risk of second primary lung cancer ranged from 3% to 6% per person-year after surgery and did not diminish over time, a finding similar to the 6% incidence of second primary lung tumors after SABR.
Toxicity
After a median follow-up of 1.6 years following SABR, the most common toxicity reported for 505 patients was pneumonitis; the rate of grade 2 or higher pneumonitis was 7%, the rate of grade 3 or higher pneumonitis was 2%, and the rate of grade 5 pneumonitis was 0.2%. The median time to onset of pneumonitis was 0.4 years. The other most common toxicities included rib fracture (3%), dermatitis (2%), and myositis (1%). Serial measurements of pulmonary function parameters showed an average decrease of 3.6% in the forced expiratory volume in 1 second and of 6.8% in the diffusing capacity for carbon monoxide within 6 months and 7 to 24 months after SABR. Changes in lung function correlated strongly with pretreatment pulmonary functions, with the largest decreases in function occurring in patients with the best pretreatment values, whereas pulmonary function was stable or even improved in patients with the worst pretreatment values. In addition, symptomatic radiation pneumonitis is uncommon following the treatment of peripheral lung tumors measuring 5 cm or smaller.
Doses to the contralateral lung predicted for the risk of pneumonitis when larger tumors are treated using a volumetric modulated arc therapy delivery technique. Limiting volumes of the contralateral lung receiving 5 Gy to less than 26% reduces risk of acute pneumonitis, and an analysis in a larger patient group indicated that both the mean dose to the contralateral lung and tumor size were strong predictors of grade 3 or higher radiation pneumonitis after treatment. The findings of this study suggested that limiting the mean dose to the contralateral lung to below 3.6 Gy was optimal.
A higher incidence of severe radiation pneumonitis has been reported for patients with preexisting pulmonary fibrosis. Patients with idiopathic pulmonary fibrosis have an increased risk for grade 3 or higher pneumonitis after both conventionally fractionated radiotherapy and chemoradiation therapy, as well as after surgical resection. Although accurate estimates of the risk of high-grade radiation pneumonitis in idiopathic pulmonary fibrosis are unknown, clinicians should be aware that interstitial lung abnormalities are found on nearly 9% of CT images among individuals over 50 years of age, with definite fibrosis in 2% in a study population of patients over 50 years old. A genetic polymorphism, the MUC5B promoter, has been found to be associated with interstitial lung disease.
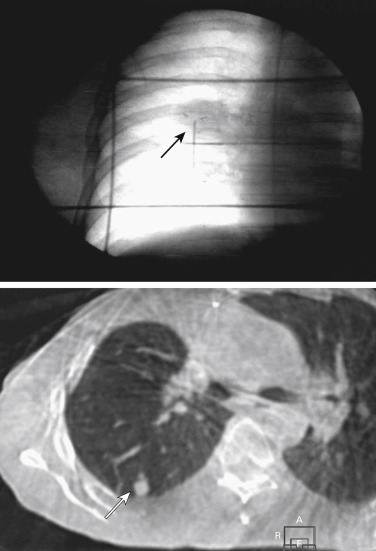
The chest wall and ribs are at risk for toxicity when tumors are located in the proximity. Current guidelines recommend limiting doses to the chest wall to 30 Gy or less, with approximately 3% of patients reporting severe chest wall pain and rib fractures ( Fig. 37.2 ) after SABR using similar constraints.
Less common toxicities after SABR include myositis, skin toxicity, and neuropathy. Brachial plexus injury can occur following SABR for apical lung tumors, with neuropathic pain developing in the shoulder or arm, motor weakness, or sensory alteration. Limiting the total dose delivered to the plexus in three or four fractions to less than 26 Gy can lower the risk of this complication. Both tracheoesophageal fistulae and esophageal perforations have been reported following SABR to vertebral or lung SABR target in the proximity of the esophagus. Careful treatment planning is necessary when performing SABR in this situation; guidelines for contouring normal organs in the thorax, as well as a summary of normal organ dose recommendations, have been published.
SABR for Central Lesions
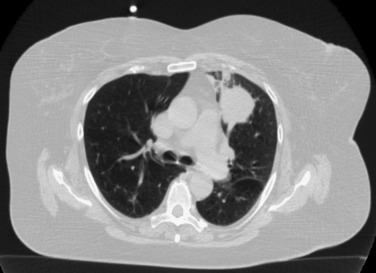
A widely used working definition for so-called central lung tumors is tumors located either adjacent to the proximal bronchial tree or located 1 cm or less from the heart or mediastinum ( Fig. 37.3 ). A higher incidence of complications has been reported after SABR for central tumors; however, in a systematic review of the literature, SABR was found to be a relatively safe and effective curative treatment, provided that appropriate fractionation schedules are used for central tumors. Preliminary analysis of the RTOG 0813 trial, which is a phase I/II study designed specifically to determine the maximum tolerated dose and efficacy of SABR for centrally located tumors, showed a 7.2% rate of grade 3 or worse toxicity using a 12 Gy × 5 fraction SABR regimen. A phase II analysis will examine efficacy rates. A similar single institution study was performed at Washington University in St. Louis, USA, which revealed that 11 Gy × 5 fractions was a safe effective dose. There was a single episode of fatal hemoptysis among the 42 patients treated. Further progress in the use of SABR for central tumors requires ongoing prospective multi-institution trials in order to establish reliable normal organ tolerance dose constraints. However, even the use of so-called risk-adapted fractionation schemes cannot completely preclude a risk of bronchial stenosis when central lung tumors are treated, but the continued use of this technique in less-fit patients is justified in light of the reported toxicity associated with surgery.
Follow-Up After SABR
Approximately 6% of patients are diagnosed with second primary lung cancers during follow-up after SABR. This finding supports the recommendation that patients treated with radical intent should be followed up for detection of treatable relapse, or the occurrence of a second primary lung cancer. While disease progression or recurrence typically occurs within the first 2 years after treatment, current and former smokers remain at elevated risk for developing second primary lung cancers beyond 2 years. A follow-up visit every 3 months to 6 months is recommended during the second and third year after SABR, with annual thoracic CT thereafter. In addition, patients with NSCLC should be offered a smoking-cessation program, as smoking cessation leads to superior treatment outcomes.
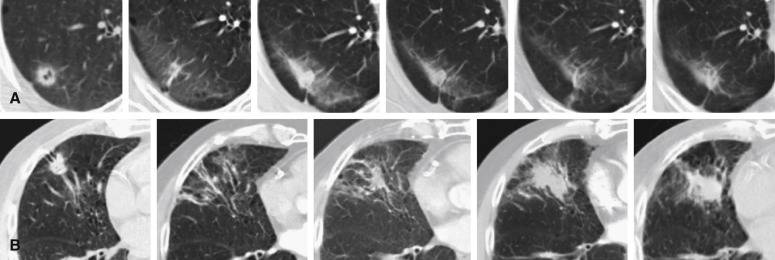
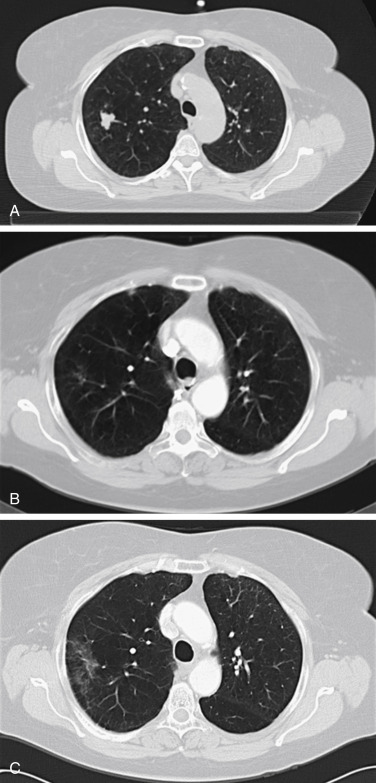
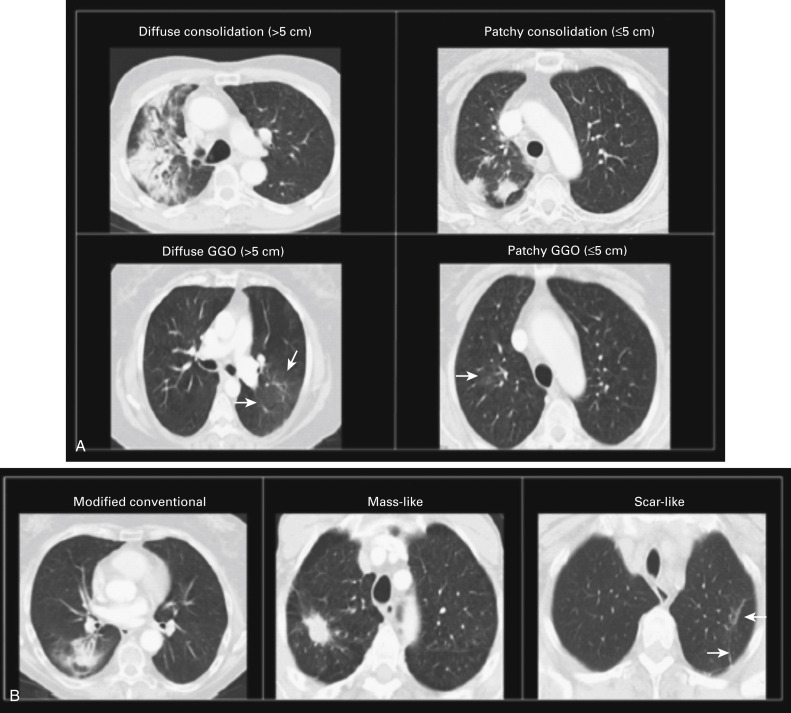
Persistent radiographic changes are common after lung SABR ( Fig. 37.4 ), with some degree of late change being nearly universal. Radiation induced lung injury surrounding the treated tumor has been found to correlate with radiation treatment isodose. A standardized classification system for benign changes has been proposed, with changes classified as acute (within 6 months after treatment) or late (more than 6 months after treatment) ( Fig. 37.5 ). Acute changes include diffuse consolidation, patchy consolidation, diffuse ground-glass opacities, and patchy ground-glass opacities; late changes include a modified conventional pattern, defined as volume loss, traction bronchiectasis, consolidation similar to changes after conventional radiotherapy (but less extensive), mass-like fibrosis, and scar-like fibrosis. Such changes often continue to evolve more than 2 years after SABR, with late changes occasionally showing mass-like effects. Multidisciplinary teams should recognize this finding in order to avoid unnecessary diagnostic procedures.
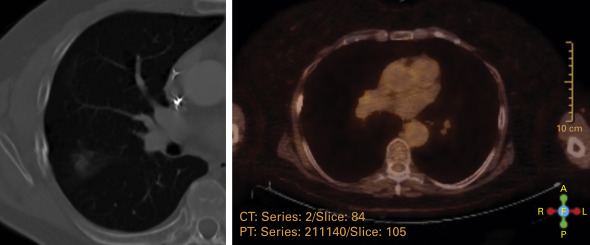
In a systematic review of the literature, so-called high-risk radiographic features associated with tumor recurrences on CT were identified. These features were (1) an enlarging opacity at the primary site, (2) sequential enlarging opacity, (3) enlarging opacity after 12 months, (4) bulging margins of the opacity, (5) loss of a linear margin, and (6) loss of air on bronchograms. The review also suggested that a maximum standard uptake value of more than 5 on FDG-PET carried a high predictive value of recurrence.
Subsequently, a blinded assessment of serial CT images of 12 patients with pathology-proven local recurrence, which were matched 1:2 to images of 24 patients without recurrence, demonstrated that all previously identified high-risk features were significantly associated with local recurrence ( p < 0.01). One additional high-risk feature—craniocaudal growth—was identified. The best individual predictor of local recurrence was opacity enlargement after 12 months (100% sensitivity, 83% specificity, p < 0.001). The odds of recurrence increased fourfold for each additional high-risk feature detected ( Fig. 37.6 ). The presence of three or more high-risk features was highly sensitive and specific (greater than 90%) for recurrence. These findings suggest that assessment of CT images after SABR for the presence of high-risk features may enable an accurate prediction of local recurrence. This information has been incorporated into an imaging follow-up algorithm for patients who are candidates for salvage therapy ( Fig. 37.7 ) in order to allow for the timely administration of curative salvage therapy.