The mechanism of cord injury is not entirely understood. The early myelopathy associated with MSCC may be due to impairment of venous drainage, leading to intramedullary vasogenic edema. When the interstitial pressure rises beyond a critical threshold, cord perfusion is compromised, resulting in necrosis. Pathologic fracture of a vertebral body and posterior displacement of bone fragments lead to mechanical cord destruction. On histopathologic examination, demyelination or necrosis of white matter is the predominant finding at the level of cord compression, whereas gray matter is relatively well preserved.13
The complex syndrome of back pain is composed of local, radicular, and referred components. Pain-sensitive structures of the spine are the vertebral periosteum, the posterior longitudinal ligament, and the synovia of the facet joints innervated by branches of segmental spinal nerves. Pain from metastatic tumor spread to the spine ensues when the cancer infiltrates the periosteum. Radicular pain results from compression or infiltration of a nerve root. Pain can also be the consequence of irritation of long tracts of the spinal cord (funicular pain) or paravertebral muscle spasm.
Micturition is frequently impaired in patients with MSCC. A brief review of the anatomy of bladder control may help to correctly interpret patients’ symptoms. Excitatory input to the detrusor muscle that promotes bladder emptying is parasympathetic and involves sacral spinal cord segments. Relaxation of the internal sphincter is transmitted through the sympathetic nervous system. Preganglionic neurons originate from thoracic and upper lumbar cord segments. The external sphincter muscle is innervated by motor neurons located in the anterior horn of the sacral cord (nucleus of Onufrowicz). Voluntary bladder control requires sensory input from stretch receptors within the bladder wall. This is transmitted to the pontine micturition center that also receives descending input from the paracentral lobule of the frontal lobe. The coordinated inhibition of internal and external sphincter muscle is mediated through the pontine micturition center. Spinal cord compression above the conus results in lack of voluntary control of micturition. Reflex emptying is possible but incomplete. When the sacral spinal cord is destroyed, the patient suffers from external sphincter insufficiency, unawareness of bladder fullness, and overflow incontinence. The mechanisms for control of defecation are similar.
Pain is the most common presenting symptom in patients with metastases involving the axial skeleton.6,8,18,19 Any back pain in a patient with cancer known to frequently seed to spine or epidural space should be considered of metastatic origin until proven otherwise. Pain ensues when the richly innervated periosteum is involved. In its early stage, it may be localized to the affected spine segment. The vertebral body is tender to percussion. Pain that results from epidural mass effect is typically exacerbated by sneezing, coughing, or the Valsalva maneuver. Because it is aggravated by the recumbent position, patients experience maximum pain intensity on awakening in the morning; they may even sleep in a sitting position. Compression of a nerve root is associated with lancinating pain in the corresponding radicular distribution provoked by Valsalva maneuver. Radicular pain in the thoracic region is usually bilateral, whereas cervical and lumbar radiculopathies are unilateral.27 Paravertebral muscle spasm caused by nerve root irritation from a metastasis results in straightening of the physiologic cervical or lumbar lordosis. Straight-leg raising (Lasegue maneuver) or, more specifically, crossed straight-leg raising (passive elevation of the contralateral, pain-free leg), exacerbates a lumbosacral radiculopathy. Referred pain may mimic a radiculopathy. Especially with intraneural tumor spread, neuropathic features (allodynia, hyperpathia, hyperalgesia) may predominate.
Neurologic symptoms typically evolve within weeks to months of the onset of back pain.15,19 Hyperacute presentation with the evolution of paraplegia within hours to days is not uncommon in bronchogenic carcinoma, while a much slower course is typical for metastases from breast cancer.13 Motor dysfunction (weakness, spasticity) is the earliest sign and occurs before sensory disturbance. Only one-third of patients report lower extremity weakness as an initial symptom. However, at diagnosis, less than one-third of patients are ambulatory.6,8,18 Typical early complaints are leg “heaviness” and difficulty climbing stairs or getting up from a chair. As the majority of malignancy-related cord compressions occur at the level of the thoracic spinal cord, most patients present with a paraparesis. The rare patient with a cervical spinal cord metastasis is expected to have quadriparesis of varying degree and, if the high cervical cord is compromised, respiratory insufficiency. Epidural progression of metastases to the upper lumbar spine results in conus medullaris syndrome with distal lower extremity weakness, saddle paresthesia, and overflow leakage from bladder and bowel.
Only few patients report diminished sensation below the level of compression at initial presentation. The level of hypesthesia is usually two to three segments below the metastatic lesion. Discrepancy of up to 10 levels above or below the lesion has been described.18 Tingling paresthesias radiating down the spine into the extremities on brisk flexion of the neck (Lhermitte sign) indicates an intrinsic or extrinsic spinal cord process. Ataxia in a patient with MSCC reflects compression of spinocerebellar pathways.
Symptoms of neurogenic bladder dysfunction are less common at symptom onset but are frequently overlooked or “rationalized” by the patient. A detailed micturition history is indispensable as patients are unlikely to report their symptoms until their compensatory mechanisms fail. New onset of nocturia or pollakisuria in the correct clinical setting should alarm the physician, and a common explanation by the patient (“I’ve been drinking a lot”) should be disregarded. Alarming symptoms of bladder dysfunction are hesitancy and urinary retention. At diagnosis, almost half of patients with MSCC are incontinent or require catheterization.8
Presence of a Horner syndrome (the combination of miosis, ptosis, and enophthalmos) indicates transforaminal progression of tumors located at the level of the cervicothoracic junction and infiltration of the stellate ganglion.
Infiltration of the lumbosacral plexus or peripheral nerves originating from it (femoral, sciatic nerve) has to be distinguished from malignant epidural compression of a root or the cauda equina. With unilateral involvement, bladder and bowel symptoms are absent; however, bilateral infiltration of plexus or nerve, giving rise to incontinence, has been seen in neurolymphomatosis and perineural spread from pelvic malignancies. Herpes zoster is encountered at spinal levels previously or concurrently affected by cancer.13,28
The cauda equina syndrome is characterized by an asymmetric painful lumbosacral polyradiculopathy, a patchy sensory deficit corresponding to multiple lumbar and sacral nerve roots, and bladder and bowel incontinence. In a patient with cancer, this syndrome raises suspicion for leptomeningeal carcinomatosis. The presence of signs and symptoms referable to intracranial disease (headache, asymmetric cranial neuropathies) facilitates the diagnosis.
Intraparenchymal spinal cord metastases and primary cord tumors are rare but may resemble epidural disease. Metastatic cord tumors predominantly arise from small-cell lung and breast cancers.29 Infectious (herpes simplex, human T-lymphotropic virus) and autoimmune myelitis are examples of not directly cancer-related myelopathies that have to be distinguished from MSCC. Predominance of transverse myelopathic features in the absence of pain is indicative of an intraparenchymal process.
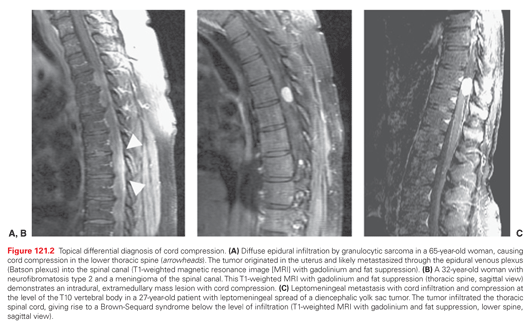
Spinal cord hemisyndromes indicate intrinsic spinal cord disease. A classic Brown-Sequard syndrome characterized by leg weakness and loss of proprioception on the side of cord infiltration and loss of pain and temperature sensation on the opposite side is rarely seen but incomplete variants exist. Leptomeningeal spread of highly aggressive tumors can lead to spinal cord infiltration, causing an overlap syndrome of extrinsic and intrinsic cord disease (Fig. 121.2C).
The mere complaint of back pain in a patient with cancer frequently does not lead to an immediate workup for vertebral metastases. Only the occurrence of more severe symptoms such as sphincter dysfunction or paraparesis sets off a comprehensive diagnostic procedure.6 Despite the availability of sensitive diagnostic tests, the average time between onset of symptoms and definitive diagnosis is still 3 months (range, 37 to 205 days). Two-thirds of this time passes after the patient reports the symptoms to a health professional.18 An interesting pattern was observed in a Scottish study. The rate of cord compression diagnosis steadily increased throughout the course of the week and reached its peak on Friday.18
With the availability of magnetic resonance imaging (MRI), the diagnosis of MSCC has been simplified. The decision to use this tool depends on the clinical evaluation. New onset or change in character of preexisting back pain in a patient with cancer or atypical back pain in the absence of a cancer history warrants measures beyond plain X-ray films and symptomatic therapy. Degenerative spine disease mostly affects the lower cervical and lower lumbar spine, the segments of largest motion. The pain waxes and wanes and responds to bed rest and symptomatic treatment with nonsteroidal anti-inflammatory agents. Pain located in the thoracic spine, progressive pain despite conservative measures, or pain aggravated by supine position should raise the suspicion for MSCC.
MRI of the entire spine is the most sensitive diagnostic test when MSCC is suspected in a patient with cancer. The study can accurately identify the level of the metastatic lesion and guide the radiation oncologist in planning the treatment field. Multiple levels of involvement present in up to one-third of patients with MSCC are recognized.20,30 Vertebral metastases without protrusion into the epidural space are detected before a potentially irreversible cord syndrome ensues. Metastases can be distinguished from other pathologic processes, involving the axial skeleton, epi- and intradural space, and spinal cord. Bacterial abscesses typically cause end-plate destruction and invasion of the disc space, whereas metastatic deposits leave the latter intact. Leptomeningeal carcinomatosis appears as nodular or linear tumor deposits in the medullary pia and along intradural nerve roots. Intradural extramedullary tumors such as meningioma or nerve sheath tumors can be easily diagnosed by their characteristic appearance and enhancement with contrast dye. Intramedullary metastases or primary tumors cause enlargement of the cord and thus can be distinguished from infectious or inflammatory myelitis that does not expand its transverse diameter.
Plain X-ray films of the spine lack sufficient sensitivity. Series of the pre-MRI era found signs of vertebral metastasis at the level of cord compression on plain X-ray films in only 80% of patients, and multiple levels of involvement were missed.19 The local extent of metastatic disease is frequently underestimated, and paraspinal tumors with transforaminal extension may be entirely overlooked.
Myelography after intrathecal injection of water-soluble contrast material with or without computed tomography (myelography, computed tomographic myelography) was the diagnostic procedure of choice in the pre-MRI era. Epidural lesions that result in complete block of the subarachnoid space obscure the extent of disease and require a second procedure with cervical or suboccipital injection of dye in order to characterize metastatic deposits rostral to the block. The study remains an option for patients in whom MRI is contraindicated.
Scintigraphic examination of the skeletal system is most useful as a screening procedure for bone metastases. Its resolution, specificity, and sensitivity are inadequate to evaluate a patient with signs or symptoms of epidural metastasis and predict the level of cord compromise.18 Myeloma may completely evade scintigraphic detection.
Positron emission tomography is likewise most useful as a staging procedure and cannot substitute for more detailed anatomic imaging techniques.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








