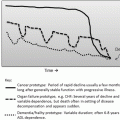
Fig. 13.1
Effect of topical estrogen therapy : Both images are from 64-year old G2P2002 women who underwent 2 vaginal deliveries. (a) Patient discontinued estrogen therapy 5 years previously. (b) Patient on estrogen continuously since menopause. Images courtesy of Dr. Murray A. Freedman © 2008
It is important to remember that age-related vaginal atrophy is a diagnosis of exclusion, and other etiologies including lichen sclerosis, lichen planus, sexually transmitted infections, and neoplasia must be considered before attributing symptoms, such as hematuria, postmenopausal bleeding, itching, or discharge, to estrogen deprivation [15]. Thorough history and physical examination are paramount to developing the correct diagnosis.
In addition to the effect on the vagina, lack of estrogen also impacts other tissues in the pelvis. Autonomic and sensory neurons in the vagina are responsive to estrogen, and treatment with topical estrogen has been shown to decrease innervation density, which may partially explain symptom improvement with estrogen therapy [16]. The female lower urinary and genital tracts are both embryologically derived from the urogenital sinus, and estrogen receptors have been found in the vagina, urethra, and bladder trigone [11]. These receptors may contribute to the impact of estrogen deprivation on lower urinary tract symptoms, and treatment with topical estrogen has been shown to improve nocturia, recurrent urinary tract infections, frequency, urgency, and incontinence, both urgency and stress urinary incontinence [11, 12].
Treatment improves symptoms of the genitourinary syndrome of menopause and can be either hormonal or non-hormonal [12, 15, 17]. Non-hormonal treatments, such as pH-balanced gels, water-based moisturizers, or hyaluronic acid, can work well for patients with few, minor complaints, whereas patients with more than two symptoms get better relief from vaginal estrogen therapy [12, 15]. Selective Estrogen Receptor Modulators (SERMs ), like ospemifene, which is an estrogen agonist in the vagina but not the endometrium, and Tissue Selective Estrogen Complexes (TSEC ), which combine an estrogen and a SERM, are effective in treating problems like moderate-to-severe dyspareunia (ospemifene) or vaginal symptoms and maturation index (Conjugated Equine Estrogens with bazedoxifene) [15, 18–21].
There are many commercially available preparations of vaginal estrogen in the USA, and all are considered safe and efficacious at the approved dose and frequency [12, 15, 22]. Delivery options such as vaginal creams, vaginal tablets, pessaries, and ovules/rings are available, and the hormones can include conjugated equine estrogens, estradiol, estriol, or promestriene [12, 15, 17]. Some conjugated equine estrogen products have been associated with slightly higher rates of side effects like bleeding, breast tenderness, and endometrial hyperplasia, but they are still considered safe and effective [12, 15, 17].
Concerns about hormone use have decreased the percentage of women using systemic estrogen therapy and have arguably been detrimental to the urogenital health of women [23]. While systemic estrogen levels are low and within the normal, postmenopausal range for women using low-dose vaginal estrogen, some studies have shown elevation in estrogen levels above pretreatment baselines, although systemic absorption decreases as the vagina becomes more estrogenic [12, 15, 17, 22, 24]. For many women, this change is likely insignificant; however, for women with a history of estrogen-sensitive cancer, particularly those taking aromatase inhibitors, vaginal estrogen is not recommended as a first-line therapy for genitourinary syndromes [12, 15, 17]. The risks and quality-of-life benefits can be discussed and balanced on an individual basis if non-hormonal treatments are insufficient for symptom relief [12, 15, 17].
While there are no long-term data to confirm endometrial safety for women with a uterus, most expert recommendations and current guidelines state that treatment with a progestin is not indicated for women using low-dose vaginal estrogen therapy [12, 17, 25]. Low-dose estrogen does not appear to increase the risk of endometrial pathology significantly and there are potential increased risks of thrombosis and breast cancer with the progestin [15]. As always, any postmenopausal vaginal bleeding should be thoroughly evaluated [25].
In spite of the prevalence of symptoms, adverse effect on quality of life, and the availability of effective treatments, vaginal atrophy is underreported [6, 9, 15, 17, 26]. Increasing awareness by asking about specific atrophy symptoms and consequently getting treatment to affected women is an important way of improving the urogenital health and general quality of life for older female patients [15].
13.3 Prolapse
Pelvic organ prolapse is a bothersome condition that has a significant negative impact on quality of life. By age 80, 12.6 % of women will undergo surgical treatment for prolapse, and the actual prevalence is even higher when symptomatic women managed non-surgically are included [3]. Prolapse is undoubtedly a multifaceted problem with many different biological, lifestyle, and other inciting factors [27]. Older age, white race, higher parity, prior hysterectomy or prolapse/incontinence procedure, obesity, frequent heavy lifting, chronic constipation, chronic coughing, and smoking have all been linked with greater risk of prolapse [27].
Symptoms of prolapse tend to be related to the most advanced portion of the prolapse and are often pelvic pressure, heaviness, or feeling a bulge. Pelvic pain and low back pain are not associated with greater degree of prolapse and many women will not experience symptoms of prolapse until the leading edge is at the hymen or beyond [27].
13.3.1 Evaluation
Use of a Sims speculum or the posterior blade of a Graves speculum can allow the examiner to inspect the anterior and posterior compartments separately, and the apex can be examined digitally or by retracting the anterior and posterior compartments simultaneously. Rectovaginal examination can also be useful in evaluation of the posterior compartment, including differentiating between rectocele and enterocele [27].
The Pelvic Organ Prolapse Quantification (POPQ ) System is widely used in the research setting as it allows for standardization of physical findings by defining the locations of points on the anterior, posterior, and apical vagina as well as genital hiatus and perineal body (see Fig. 13.2) [28]. While the entire POPQ does not necessarily need to be performed in the clinical setting, identification and recording of key attributes including the leading edge of the anterior, apical, and posterior compartments is important and clinically relevant [27]. Stages of prolapse are 0-IV based on the leading edge, e.g. most severe portion, of the prolapse with 0 being no prolapse (apex is within 2 cm of total vaginal length) and stage IV being total eversion within 2 cm of the total vaginal length [28].
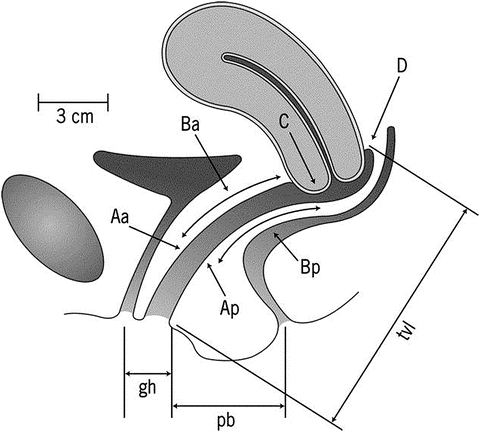

Fig. 13.2
Diagrammatic representation of the pelvic organ prolapse quantification system for staging prolapse by physical examination findings, showing the 6 sites (points Aa and Ba anteriorly, points Ap and Bp posteriorly, point C for the cervix or apex, and point D for the cul-de-sac), genital hiatus (gh), perineal body (pb), and total vaginal length (tvl) used for pelvic organ prolapse quantification. Reprinted from Weber AM, Richter HE. Pelvic Organ Prolapse. Obstet Gynecol 2005;106:615–34; modified from Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JOL, Klarskov P, Shull BL, Smith RB. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10–17
Pelvic organ prolapse presents along a spectrum from asymptomatic women with minimal anatomic findings to severely bothered patients with total vaginal vault prolapse or uterine procidentia. Generally speaking, asymptomatic patients do not require treatment, and surgery should not be performed unless the patient’s symptoms warrant the potential risks of intervention [27]. Whereas in the past, some thought that early surgical treatment of prolapse may prevent progression, observation of a cohort treated for stress urinary incontinence showed that only 2 % of asymptomatic women with stage II prolapse had any anatomic worsening of their disease and none underwent surgical treatment in the 5- to 7-year follow-up period [29].
13.3.2 Non-Surgical Treatment
For women who are symptomatic, non-surgical management options include pessaries and pelvic floor muscle training. These options can be very appealing for women who have less bothersome symptoms or significant surgical risk, but they should be considered and offered to all women. Adjunct therapies to optimize other aspects of disease should also be considered including lifestyle changes, like weight loss, as well as treatment of chronic constipation and defecatory dysfunction [27].
The pessary is a very useful device for the non-surgical treatment of prolapse, and most women can be successfully fit. Of Medicare beneficiaries with a prolapse diagnosis, 11–13 % were treated with a pessary [30]. While there are many different designs and sizes, the two main categories are support and space-filling, and the ring with support and Gellhorn pessaries are probably the most useful in each of these respective categories (see Fig. 13.3) [27, 31]. The ring with support is often the first choice because of its ease of use and ability for many patients to remove, clean, and manage it themselves. For women who cannot retain the ring with support, a Gellhorn is often an effective option, but it tends to require provider visits for removal and cleaning [31]. Women who are sexually active and use a pessary should be able to remove and reinsert the pessary themselves since most, if not all, pessaries are not compatible with vaginal intercourse [31]. Vaginal epithelial health is an important consideration with pessary use, and vaginal estrogen therapy should be considered if needed, although many women may not require it. Periodic inspection of the vagina for abrasions and ulcerations is essential, and compliance with follow-up is key to identifying problems before they result in severe complications [27, 31]. While there are no data-driven guidelines on follow-up intervals, typically every 3–6 months for a patient unable to remove her own pessary is reasonable, and that can be extended as long as 1 year for a woman who is able to remove and clean the pessary frequently herself [31, 32]. Usually minor abrasions or ulcerations can be resolved by leaving the pessary out and applying vaginal estrogen cream for several weeks. More significant complications, such as fistula formation, typically only result from extended neglect [31]. Vaginal discharge and unmasking of occult stress urinary incontinence can also be bothersome side effects of pessary use [31, 33].
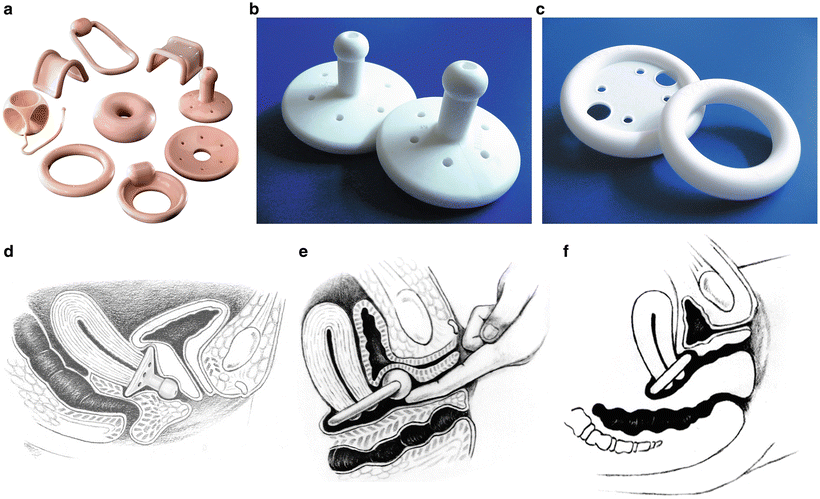

Fig. 13.3
Pessaries : (a) an assortment of pessaries, (b) Gellhorn pessaries, (c) ring and ring with support pessaries, (d) Gellhorn pessary in position, (e) Ring with incontinence knob pessary in position, (f) Ring pessary in position. a, d, e, f, Photographs provided by CooperSurgical Inc; Images b, c, Photographs provided by BIOTEQUE AMERICA, INC
Pelvic Floor Muscle Training (PFMT ) can be effective in reducing symptoms for women with mild to moderate (usually stage I to II) prolapse [31]. This treatment usually involves working on isolation of pelvic floor muscles and doing exercises which strengthen and improve muscle bulk. Studies have shown both symptomatic and anatomic improvements with PFMT for patients with stages I, II, and III prolapse [31, 34]. Success of these treatments is likely dependent, however, on having motivated patients who are willing to comply with the exercise program.
For women who desire more than non-surgical management for their prolapse symptoms, there are many surgical treatment options available. These options include both obliterative and reconstructive procedures.
13.3.3 Surgical Treatment
Obliterative procedures, such as the Le Fort colpocleisis with levator plication and high perineorrhaphy, have many advantages for women who do not desire preservation of the ability for vaginal intercourse. These procedures tend to be shorter and less morbid than reconstructive repairs and are highly effective [27, 35–39]. Success rates range from 91 to 100 %, which is outstanding for efficacy of prolapse repair [38]. Patient-centered outcomes are excellent with 90–95 % of patients experiencing improved quality of life, satisfaction with outcome, and willingness to recommend the procedure to others [35, 36, 38]. Post-surgical regret, although very uncommon (approximately 5–10 %), is not zero risk [27, 35, 40].Urinary tract infection is the most common postoperative complication, and women who underwent simultaneous colpocleisis and midurethral sling do not have increased complications in the immediate postoperative period [37].
Reconstructive repairs can be performed vaginally or abdominally, and can be performed with native tissue or using augmentation with mesh, fascia, or biologic grafts. Minimally invasive options include the vaginal, laparoscopic, and robotic approaches. Currently, no clinical trials have definitively shown which methods of prolapse repair are the most effective.
Vaginal native tissue or “traditional” repairs can be performed in all three compartments: apical, anterior, and posterior. The two most common methods used for apical support are the high uterosacral ligament suspension and sacrospinous ligament fixation (see Figs. 13.4 and 13.5) [41–44]. These methods were compared head-to-head in the Pelvic Floor Disorders Network’s Operations and Pelvic Muscle Training in the Management of Apical Support Loss (OPTIMAL) trial and were shown to have similar outcomes for anatomic and functional success as well as adverse events [44]. The types of adverse events did differ, however, with ureteral obstruction being more likely with uterosacral suspension and buttock pain being more likely in the sacrospinous suspension groups. Usually ureteral obstructions can be identified on intraoperative cystoscopy and can be resolved without any lasting repercussions. The buttock pain from sacrospinous suspension generally resolves without intervention in most patients by 6 weeks postoperatively, however a small subset (<5 %) may require interventions including physical therapy or trigger point injections for the pain [44, 45]. With the strict definition of success used for the OPTIMAL trial, approximately 60 % of patients were considered to have successful outcomes, 5 % of patients required repeat surgical treatment.
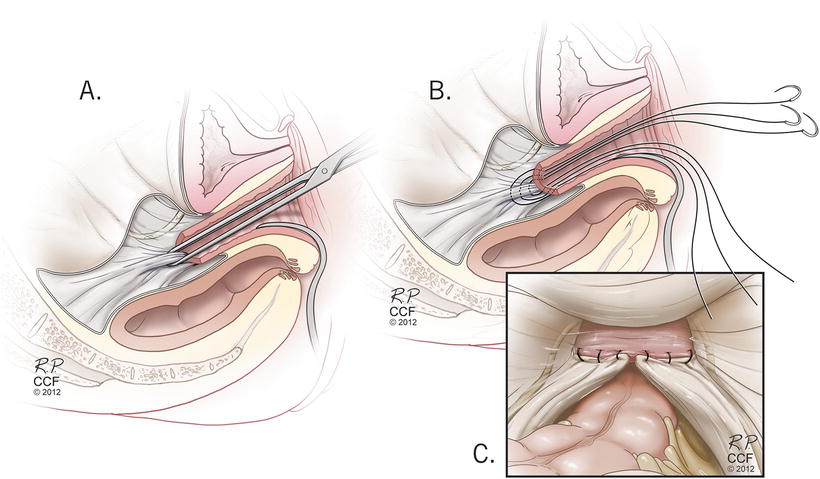
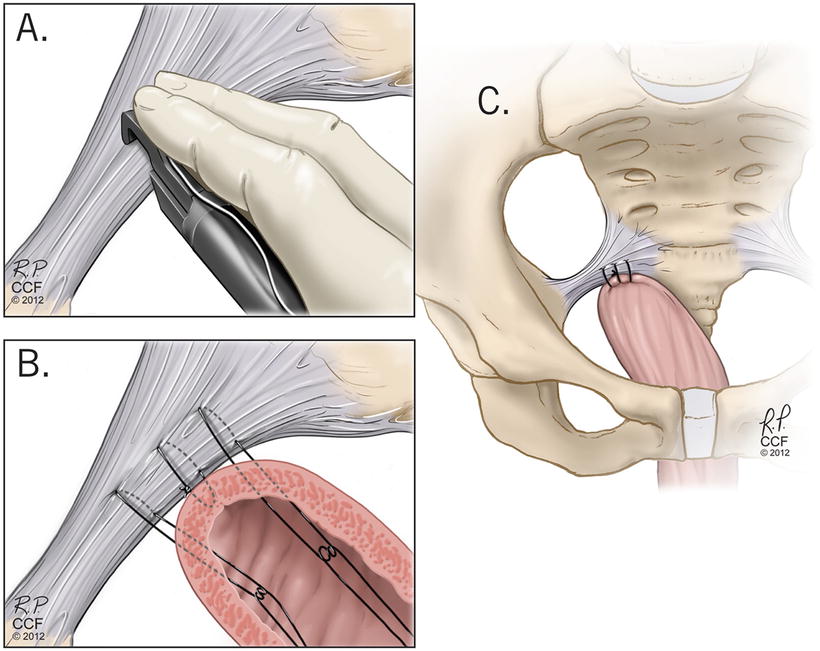

Fig. 13.4
High uterosacral ligament suspension technique . Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2004–2015 All Rights Reserved

Fig. 13.5
Sacrospinous ligament suspension . Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2004–2015 All Rights Reserved
Suspension of the apex is critical to the success of prolapse repairs. In addition to appropriate apical suspension, other defects should also be addressed including enterocele, cystocele, and rectocele. Enterocele can be repaired with cephalad purse-stringing of the enterocele sac, with or without excision of the sac, and reapproximating the anterior and posterior apical vaginal connective tissue [27]. Anterior colporrhaphy is the preferred native tissue repair for the anterior compartment defects, but paravaginal repairs can also be considered when appropriate for surgeons with sufficient expertise [27]. Posteriorly, traditional colporrhaphy is recommended with perineorrhaphy as needed. Care must be taken not to overcorrect or narrow the vagina, which could cause pain or worsened sexual function [27].
Vaginal repairs augmented with mesh have been a recent topic of controversy. In light of apparent failure rates with native tissue repairs, there was keen interest in the possibility of improved results with mesh augmentation. Popularity of mesh augmentation grew more quickly than the data supporting its use, and concerns about safety and efficacy were raised [46, 47]. Many of the original vaginal mesh products have been discontinued, and those that remain are being rigorously investigated to assess their clinical outcomes.
From the existing data, it appears that mesh augmentation may improve outcomes in the anterior compartment, but further study is needed [46–48]. There are not currently data to support the use of vaginal mesh for apical and posterior support [46, 47, 49, 50].
Abdominal sacrocolpopexy , which can be performed open, laparoscopically, or robotically, is a procedure in which a graft is used to pull the vagina up to the sacrum, and it has been considered the most durable prolapse repair option (see Fig. 13.6). Longer-term studies have shown, however, that even with sacrocolpopexy, success rates decrease over time [51]. At 5 years, nearly a third of women in the eCARE trial met treatment failure criteria, but only 5 % had undergone a repeat procedure. Additionally, mesh exposure rate was about 10 % and exposures continued to occur throughout the extended study period. Minimally invasive abdominal sacrocolpopexy can be performed laparoscopically or robotically and has similar prolapse outcomes as an open abdominal procedure [52, 53]. Minimally invasive procedures have longer operating times but less blood loss and shorter hospital stays than open procedures [52, 54]. When comparing laparoscopic and robotic modalities, laparoscopy has been shown to offer decreased cost, shorter operative time, and less pain at 1 week postoperatively [55]. One study also showed less blood loss, lower rate of bladder injury, and decreased reoperation rate with laparoscopic as compared to robotic sacrocolpopexy [56].
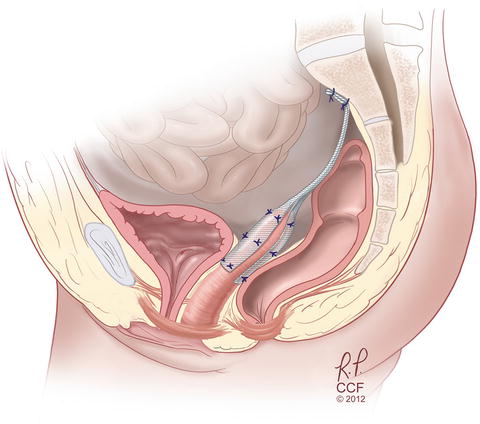

Fig. 13.6
Sacrocolpopexy with mesh attached to anterior and posterior vagina as well as sacrum. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2004–2015 All Rights Reserved
Older patients undergoing urogynecologic surgery have been shown in some studies to have similar outcomes as younger women [57, 58]. Much like with midurethral slings, however, some studies did find higher rates of complications for older patients [59]. Even so, the overall rates of complications are low, and chronological age should not be the only factor in surgical decision making.
13.3.4 Urinary Function
Urinary incontinence (UI ) is a common pelvic floor disorder which affects 49.2 % of adult women, and increases to above 60 % prevalence in women over age 70 [60]. Prevalence of incontinence starts off gradually in young adults, reaches a peak in mid-life, and climbs steadily in the older population [61, 62]. While the overall prevalence increases with age, the distribution of incontinence types changes from more stress incontinence in younger women to more urgency and mixed incontinence in older women [60]. Older women also tend to have more severe incontinence than younger women [60, 63]. Urinary incontinence is not considered a normal part of aging and has a huge impact on patients’ lives [64]. UI has been associated with functional decline, fall risk, nursing home placement, depressive symptoms, and frailty [64, 65].
Even when incontinence is significantly bothersome, many women do not seek care [64, 66]. Patients are often reticent to mention these issues to providers, who must initiate the conversation.
The burden of disease for urinary incontinence is significant—economically and emotionally. UI severity has been associated with major depression, medical comorbidity, and decreased quality of life, particularly in those with nighttime and coital symptoms or comorbid fecal incontinence [66–70]. The financial cost of UI is estimated at more than $16 billion in 1995 dollars, $7.6 billion of which was for women over age 65 [71]. In spite of a 15 % decrease in the cost per capita, Medicare costs for female beneficiaries nearly doubled from 1992 to 1998, due to an increase in the number of patients requiring treatment [72].
13.3.5 Definitions
The terminology for urinary incontinence was standardized by the International Continence Society [73–78].
Urinary Incontinence
The complaint of any involuntary leakage of urine.
Stress Urinary Incontinence (SUI )
The complaint of involuntary leakage on effort or exertion, or on sneezing or coughing.
Urinary Urgency
The complaint of a sudden, compelling desire to pass urine which is difficult to defer.
Urgency Urinary Incontinence (UUI )
The complaint of involuntary leakage accompanied by or immediately preceded by urgency.
Mixed Urinary Incontinence (MUI )
The complaint of involuntary leakage associated with urgency and also with exertion, effort, sneezing, or coughing; applies to people with symptoms of both SUI and UUI.
Overactive Bladder Syndrome (OAB )
Urinary urgency, with or without urgency incontinence, usually with increased daytime frequency (e.g., the complaint by the patient of voiding too often by day) and nocturia (complaint of waking up once or more at night to void) in the absence of urinary tract infection or other obvious pathology.
Other pertinent types of incontinence include [77]:
Functional Incontinence
Untimely urination due to physical disability, lack of access to a toilet, or problems in thinking that prevent a person from reaching a toilet.
Overflow Incontinence
Unexpected and near continuous leakage of small amounts of urine because of a distended bladder which is not emptying properly; the etiology is from either outlet obstruction or inadequate detrusor contraction. Causes include neurologic impairment, fecal impaction, and medication adverse effects.
13.3.6 Impact of Age
Age-related changes are important contributors to urinary incontinence in older patients, but they can be difficult to delineate from comorbidities and confounding factors, like parity [79]. There are, however, many age-related changes in the anatomy and physiology of the lower urinary tract (LUT) [65, 79, 80]. Detrusor contractility weakens, urethral closure pressure decreases, urethral blood flow and vascular density decrease [65, 79, 80]. Older patients also tend to have more detrusor overactivity (DO), higher post-void residual (PVR) volume, lower volume voids, and decreased flow rate [79]. These changes accompany the previously discussed increases in urgency UI, frequency, and nocturia [79]. Additionally, medical comorbidities, neurologic/psychiatric status, functional and environmental issues, and medications impact UI and make it a multifactorial geriatric syndrome [65, 79]. This complexity is clinically relevant as addressing those components may improve symptoms without any other interventions [79, 81].
Other risk factors for UI include female gender, white race, and elevated body mass index (BMI) [60, 82, 83]. Hysterectomy, smoking, thyroid disease, depression, decreased physical activity, arthritis, diabetes, and childbirth have also been linked [60, 82, 83]. Both vaginal and cesarean delivery have been associated with an increased risk of UI, but the impact of parity is stronger in younger women and appears to dissipate by age 65 [84, 85]. Neurological status, chronic cough, menopause, collagen integrity, and medication use are also important factors [82]. Persistence of UI has been associated with increased age, white race, higher parity, elevated BMI, decreased physical activity, type 2 diabetes, stroke, and hysterectomy, yet the greatest increased odds of UI were associated with older age, white race, and obesity [86].
13.3.7 Evaluation
In the initial evaluation of UI, patient history is essential to differentiate the type of incontinence (SUI, UUI, MUI, overflow), and urinalysis is also recommended to rule out hematuria, pyuria, bacteriuria, and glycosuria [64]. Physical examination is useful for evaluation of anatomy, atrophy, pelvic floor tone, strength, and coordination. Post-void residual (PVR ), simple cystometrics, and complex urodynamics can also be useful but are usually not needed in the initial evaluation of most patients [87].
13.3.8 Non-Surgical Treatment
13.3.8.1 Contributing Factors
Like other geriatric syndromes, UI often has more than one cause, and successful treatment often entails addressing several of these factors [65]. For many older women, especially those who are frail, simply addressing contributing factors regardless of UI type (SUI, UUI, MUI) will improve bladder control. Contributing factors include: ensuring there is adequate access to toilets which may mean improving the patient’s mobility or adapting the environment; and if the patient is cognitively impaired, recommending prompted toileting. Prompted toileting differs from scheduled toileting because the patient is asked if they need to use the toilet on a schedule (typically every 2–3 h) but regardless of the response (yes or no) she is taken to the toilet and praised if able to void. Other contributing factors include comorbid disease and medications. Medical conditions that contribute to UI and may require referral to a primary provider to optimize treatment include: heart failure, chronic obstructive pulmonary disease, and chronic cough. Many medications contribute to UI (see Table 13.1) and should be reduced or minimized.
Table 13.1
Medications commonly associated with urinary incontinence
Medication/Class | Adverse effects/Comments |
|---|---|
ACEa inhibitors | Cough (stress UI) |
Alcohol | Frequency, urgency, sedation |
α Adrenergic agonists | Outlet obstruction |
α Adrenergic blockers | Stress leakage |
Anticholinergics | Impaired emptying, constipation |
Cholinesterase inhibitors | Increased uninhibited contractions |
Calcium channel blockers | Impaired detrusor contraction |
Estrogen (oral, transdermal) | Stress and mixed UI |
GABAb-ergics (gabapentin, pregabalin) | Edema, nocturnal diuresis |
Loop diuretics | Polyuria, frequency, urgency |
NSAIDsc/thiazolidinediones | Edema, nocturnal diuresis |
Sedative hypnotics | Sedation, delirium, immobility |
Opioid analgesics | Constipation, sedation, delirium |
Antipsychotics | Anticholinergic effects, sedation |
13.3.8.2 Urgency and Urgency Incontinence
Urinary urgency, overactive bladder syndrome, and urgency incontinence become increasingly prevalent with age and have a negative impact on quality of life [60, 88]. In many patients these irritative symptoms persist and necessitate management as a chronic disease process rather than as an acute illness [89]. First line management options include lifestyle modification and behavioral therapy; and then adding medications when symptoms are not adequately controlled.
Lifestyle modifications involve changing habits that may be contributing to urinary urgency or incontinence. Limiting caffeine, which is both a diuretic and a bladder irritant, discouraging extremes of fluid intake (too much or too little), and restricting fluid intake several hours before bedtime can be helpful [64, 88]. Constipation that places pressure on the urethral sphincter (obstruction) or places pressure on the bladder should be treated [65]. Smoking causes chronic cough and patients should be encouraged to quit. Studies in bariatric patients have shown that even a 5 % weight loss can bring a significant improvement in UI, with more weight loss conferring even greater benefit [90, 91].
Behavioral therapy involves teaching the patient techniques to reduce urgency and incontinence episodes. These can include isolating and strengthening appropriate muscle groups with Kegel exercises, learning stress strategies, urge suppression techniques like “freeze and squeeze,” and using voiding schedules to increase the amount of time between voids [64]. Behavioral techniques can be very effective but do require a cognitively intact and motivated patient [64, 88, 92].
Antimuscarinic medical therapy , including oxybutynin, tolterodine, solifenacin, darifenacin, fesoterodine, and trospium can be effective, but side effects, cost, and drug interactions must all be considered [64]. The maximum dose of trospium, solifenacin and fesoterodine must be reduced for many older women based on creatinine clearance which frequently declines with age. Due to their anticholinergic properties (which inhibits detrusor contractions), antimuscarinics have significant side effects which contributes to low adherence (less than one third) one year after initiation of antimuscarinic therapy [93]. Side effects include dry mouth, constipation, blurry vision, and the potential for cognitive impairment. Cognitive side effects are a significant concern in the older population, particularly in patients who may already have some level of cognitive impairment. Most of the antimuscarinics have not been shown to cause impairment, however several studies have demonstrated cognitive changes with oxybutynin [88, 94–98]. When possible, use of extended release antimuscarinics is preferred over immediate release as the longer acting formulations have better efficacy with fewer side effects [99]. The impact of side effects on chronic issues like cognitive impairment, constipation, dry mouth, and mobility must be considered before starting antimuscarinic therapy in any patient, but especially in an older patient.
In addition to antimuscarinics, mirabegron, a β3-adrenoceptor agonist, has been shown to be effective and well tolerated in the older population with hypertension being the most common adverse effect; blood pressure should be monitored during initiation of therapy [100]. Other reported adverse events in mirabegron treated patients include headache, nausea, dizziness, and tachycardia (including atrial fibrillation). Mirabegron is renally excreted and the maximum dose must be reduced if creatinine clearance is less than 25 ml/min. Because it is not anticholinergic, the side effect profile of mirabegron may be preferable for some patients. Vaginal estrogen treatment can also improve lower urinary tract symptoms including urgency, frequency, nocturia, and incontinence, so treatment of vaginal atrophy should be considered [12, 64, 101].
13.3.9 Procedure-Based and Surgical Treatment
When urgency symptoms are refractory to first line therapy, behavioral therapy, lifestyle interventions, pharmacotherapy, and combinations thereof, other, more invasive options may be considered. These therapies include the neuromuscular toxin, Botulinum, and neuromodulation of sacral and tibial nerves.
Percutaneous tibial nerve stimulation (PTNS ) involves using a small needle inserted near the ankle to stimulate the posterior tibial nerve [102]. These 30-min stimulation treatments are performed weekly for 12 weeks and additional treatments can be repeated as needed [102]. This technique has been shown to improve OAB symptoms up to 24 months [102].
Sacral neuromodulation (SNM ) involves a staged procedure where a lead is placed into the S3 foramen. Test stimulation is typically performed for 2 weeks, and if the patient has at least a 50 % improvement in symptoms, a permanent neurostimulator is implanted and connected to the lead [103, 104]. The neurostimulator provides continuous stimulation of sacral nerves to modulate neural signals to and from the bladder, anal sphincter, and pelvic floor (see Fig. 13.7) [105]. SNM is FDA approved for the treatment of OAB, urgency UI, non-obstructive urinary retention, and fecal incontinence (see section to follow), and appears to be as safe and effective in older patients as younger ones [64, 103, 104, 106].
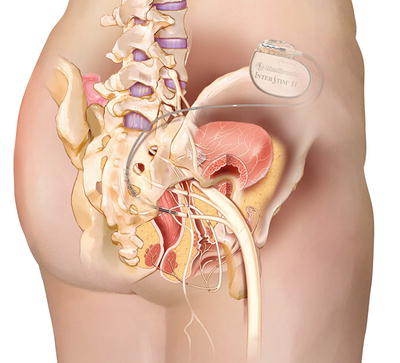

Fig. 13.7
Sacral neuromodulation device —permanent placement. Reprinted with the permission of Medtronic, Inc. © 2014
Botulinum toxin is an FDA-approved treatment for refractory OAB, which has been shown to improve symptoms and quality of life [107–109]. Botulinum toxin works at the presynaptic cholinergic junction by inhibiting the release of acetylcholine and thus causing temporary detrusor muscle paralysis [110]. It is administered cystoscopically by injecting the toxin into multiple points in the detrusor or suburothelially (see Fig. 13.8) [109, 111]. Potential adverse events include urinary retention and urinary tract infection. Patients that receive this therapy must be willing to self-catheterize if needed [108]. Botulinum toxin has been shown to be effective in the older population [112]. The Refractory Overactive Bladder: Sacral NEuromodulation vs. BoTulinum Toxin Assessment (ROSETTA) trial, a comparative effectiveness trial between Botulinum toxin and SNM for patients with refractory OAB, is currently in follow-up [113].
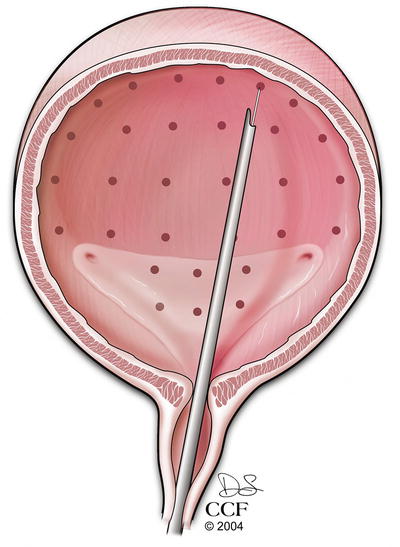

Fig. 13.8
Sites of Botulinum toxin injection during cystoscopy. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2004–2015 All Rights Reserved
13.4 Stress Urinary Incontinence
Stress urinary incontinence (SUI ) affects 15–20 % of women over the age of 65 [60, 62]. It is very costly from an economic perspective [114, 115] with annual out of pocket costs per woman at nearly $750 (in 2006 dollars) [114].
13.4.1 Evaluation
Evaluation for SUI can be straightforward with a good history and physical examination. Leak with Valsalva maneuver on exam or simple cystometrics using a bladder fill and cough stress test can be sufficient, and complex urodynamic testing is not needed for women with uncomplicated, demonstrable SUI [116].
13.4.2 Non-Surgical Treatment
Conservative management options for SUI include behavioral therapy and urethral or vaginal inserts [117]. Some women have symptom improvement with continence pessaries, continence tampons, or urethral inserts, but the cure rates are lower than with behavioral therapy [117, 118]. Pelvic floor muscle training and bladder training have been shown to improve objective and subjective cure rates for SUI and are a good initial option for treatment [117]. If a woman does not improve with conservative therapy, she can consider more invasive options.
13.4.3 Surgical Treatment
SUI can be managed surgically with procedures such as polypropylene midurethral sling placement (Fig. 13.9), pubovaginal sling, Burch colposuspension, and periurethral bulking. Studies have shown trends with an increase in the number of total incontinence procedures performed per year in the USA as well as a shift from inpatient to outpatient procedures with the advent of the synthetic midurethral sling [119, 120]. These increases are notable in women over age 52 [121]. Women over age 75, however, do not appear to be getting the same treatment as rates of polypropylene midurethral sling in this population have increased much more slowly than in younger women [122]. This disparity may stem from concerns regarding surgical complications in older patients with more comorbidities or questions regarding successful outcomes. In spite of comprising a significant proportion of the population suffering from UI, older women have been historically under-represented in clinical trials for SUI surgery [123]. More recently, however, many different studies have tried to evaluate the surgical treatment of SUI in older women [115, 124–148].
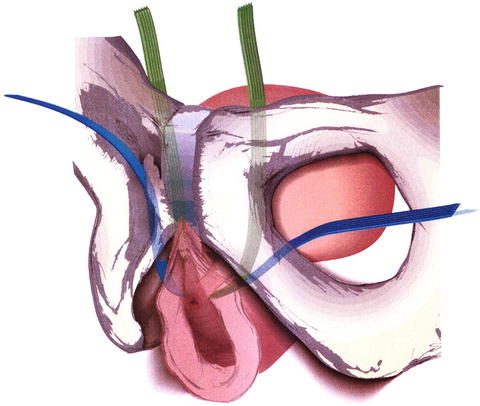

Fig. 13.9
Retropubic (green) and transobturator (blue) midurethral slings. From Retropubic versus Transobturator Midurethral Slings for Stress Incontinence, Richter HE, Albo ME, Zyczynski HM, et al., Volume 362, Supplement Page 14, Copyright © 2010 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society
Results from studies of midurethral slings in older women have been inconsistent, which may be in part due to the heterogenous study populations and varied definitions of success. Some studies show greater risk of voiding dysfunction, outlet obstruction, de novo urinary urgency, urinary tract infection, need for catheterization, need for division of sling, and perioperative medical complications with lower rates of cure and satisfaction after midurethral sling in older patients, whereas others show no differences [115, 124, 125, 128–134, 136–138, 140–143, 145, 146, 148]. Older patients may also be more symptomatic than younger ones, and two studies showed that when differences in preoperative symptom bother were considered, age did not influence quality of life outcomes postoperatively [115, 124]. One prospective, randomized clinical trial comparing immediate midurethral sling versus expectant management for 6 months in older women found a significant improvement in satisfaction, symptoms, and quality of life in the immediate surgery group [147]. Another study showed that urethral hypermobility was an important predictor of midurethral sling treatment success in older women [139]. In summary, the overarching theme of the results is that older women do significantly benefit from midurethral slings and have improved quality of life postoperatively, although their improvements may be less pronounced than in younger women.
The pubovaginal sling (PVS ) using autologous rectus fascia is another option for older patients [144, 149, 150]. Age has not been associated with worsened outcomes for PVS, however, menopausal status has [150]. At 2 years postoperatively, one study showed good short-term outcomes for PVS with 85 % of patients improved and satisfied, and another, smaller study showed 100 % of 19 geriatric women with resolved SUI [149, 151]. Long-term results from SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) were less promising for PVS with only 27 % continence at 7 years [150].
Treatment with Burch colposuspension was also studied in the SISTEr trial, which found that while patients who underwent the Burch procedure had lower success rates (38 % at 2 years to 13 % at 7 years) than those who underwent PVS they also had fewer urinary tract infections, less difficulty voiding, and less postoperative urgency incontinence [150, 152]. A sub-analysis of the older patients in the trial revealed that older women had similar perioperative outcomes and worse 2-year outcomes than younger women [144].
Periurethral bulking is another option for SUI treatment that is typically used either as a primary treatment in a patient who is a poor surgical candidate or as a secondary procedure after failure of another procedure. Two studies have evaluated bulking agents after failed midurethral sling and found cure rates of 35–60 % with few complications [153, 154]. Even with these modest success rates, one study showed 77 % of patients were satisfied with the treatment and another showed negative pad tests (no leakage on a protective undergarment pad) in more than 70 % of patients [154, 155].
13.5 Fecal Incontinence
Fecal incontinence (FI ) is defined as the unintentional loss of liquid or solid stool and anal incontinence (AI) includes the leakage of gas [156]. Estimating the number of people affected by this condition is difficult because only one third of patients discuss their incontinence with their physicians [157]. Fecal incontinence is common with prevalence rates ranging from 7 to 15 % in community-dwelling US populations [156]. The prevalence of FI is higher among care-seeking populations, home care populations, and adults in long-term care facilities [158]. In a study of community dwelling adults over the age of 65, the rate of FI over 4 years was 17 %. Controlling for age, comorbidity, and body mass index, significant independent risk factors for incident FI in women were white race, depression, chronic diarrhea, and urinary incontinence [159]. Other risk factors for FI are listed in Table 13.2 [160].
Table 13.2
Risk factors for fecal incontinence
Anal • Injury • Fistula • Rectal prolapse • Hemorrhoids • Anal carcinoma • Perianal infection • Congenital Rectal • Proctitis • Rectal carcinoma • Rectal infection Neurological • Central nervous system (stroke, dementia, spinal cord injury, tumor, multiple sclerosis, cauda equina) • Peripheral nervous system (pudendal neuropathy, diabetes mellitus) Functional • Fecal impaction • Diarrhea • Irritable bowel syndrome • Physical disabilities • Psychiatric disorders • Metabolic, medication, malabsorption |
13.5.1 Evaluation
A thorough history and physical examination is essential to establishing the diagnosis of FI and tailoring treatment options. The history should include duration of symptoms, frequency of incontinence, time of day, quality of stool, control of flatus, frequency of bowel movements, constipation or diarrhea, use of pads, and impact on quality of life. Consistency of lost stool may correlate with the severity of incontinence since solid stool is easiest, liquid stool more difficult, and flatus most difficult to control [161]. Thus those patients with loss of solid stool have the most severe incontinence. A thorough obstetrical history should also be obtained including number of vaginal deliveries, weight of babies delivered, use of forceps, and significant tears, repairs, or episiotomies.
The physical examination should start with the inspection of the anal verge area looking for any scars or deformities. The patient should be asked to squeeze to simulate holding in a bowel movement to see if there is uniform contracture of muscles. Making the patient strain, as if having a bowel movement, can show perineal descent, hemorrhoids, vaginal prolapse, or even rectal prolapse [161]. Innervation can be crudely checked by touching the perineal area with a Q-tip and monitoring for an anal wink and also with pinprick sensation.
A digital rectal examination should be performed to check for masses, blood, fistula, or rectocele [162]. During the rectal examination baseline tone represents the internal anal sphincter and the patient should be asked to squeeze for assessment of the external anal sphincter. The accuracy of digital examination is operator dependent but overall, rectal exams have been proven as reliable as anal manometry in assessing anal resting and squeeze tone [163]. The reported positive predictive value of digital examinations to identify low resting and squeeze pressures by experienced clinicians was 67 and 81 %, respectively [164, 165].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree