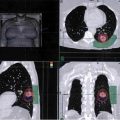
A 65-year-old male had a 4-cm lung mass found on positron emission tomography (PET); it was biopsied by core needle. The patient smoked 2 packs of cigarettes per day and has chronic obstructive pulmonary disease (COPD) and hypertension (HTN). The PET reveals positive mediastinal lymph nodes. Magnetic resonance imaging (MRI) of the brain is normal. The pathology shows small cell lung cancer (SCLC). The patient’s performance status is good, and his basic laboratory tests are normal. Learning Objectives: 1. What is the most common chemotherapy doublet for locally advanced SCLC? 2. What is the difference between cisplatin and carboplatin in regard to treatment for SCLC? 3. What treatment options are available for SCLC other than cisplatin or carboplatin plus etoposide? 4. How often should locally advanced patients be scanned during treatment? 5. How are disease relapses classified in SCLC? Small cell lung cancer (SCLC) is the fastest growing solid malignancy so it is not surprising very few patients present with early stage disease. More often, they are diagnosed at the locally advanced or metastatic stage. These patients typically present with cough and dyspnea, but depending on the extent of disease, they can also have weight loss, bony pain, debility, or even neurologic deficits. Imaging usually reveals a large hilar mass and bulky mediastinal lymphadenopathy. Though SCLC is very sensitive to chemotherapy with rapid initial response, the disease most likely will recur. The incidence of SCLC overall has been decreasing, but the incidence in women has been rising. It is often centrally located and strongly associated with smoking, so smoking cessation should always be discussed with patients. Those already diagnosed with SCLC who continue to smoke are at risk for greater toxicities with treatment and shorter overall survival.1 Unlike other solid malignancies, SCLC often is not classified using the traditional American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) staging. Instead, it is described by the Veterans Administration (VA) scheme in which SCLC is separated into only two groups: (1) limited stage, in which disease is only present in the ipsilateral hemithorax, and (2) extensive stage, wherein it has spread beyond the ipsilateral hemithorax. However, classifying patients using the TNM staging helps to define those who would be candidates for surgery and radiation, such as those with T1-2,N0. For the purpose of this book, SCLC is described as early stage, locally advanced, and metastatic. Locally advanced in this case refers to stages IIB-IIIC (T3-4,N0,M0; T1-4,N1-3,M0). Due to the aggressive nature of SCLC, many patients present with extensive disease and subsequent disease-related debility. When deciding treatment regimens in general, performance status is a key factor for consideration as it predicts how well the patient will tolerate treatment. However, SCLC patients are the exception to this rule. As mentioned, SCLC is very sensitive to chemotherapy. Patients often have some response after 1-2 cycles, in terms of not only disease response but also improvements in symptoms and functionality. A retrospective study found that 21% of patients with performance status 2 had improved performance status and prolonged survival with salvage topotecan.2 Another study showed patients with performance status of 3 or 4 who received chemotherapy had improved median survival compared to the large majority of patients who did not receive treatment (67%).3 Systemic therapy is a key component of treatment for all stages of SCLC. For patients with locally advanced disease, treatment is often with concurrent chemoradiation. Cisplatin/etoposide is the most commonly used doublet for treating SCLC, which used to be treated with alkylator/anthracycline-based regimens until the current regimen was proven to be superior. A meta-analysis of trials using a cisplatin-containing regimen versus a regimen without it found that the groups treated with cisplatin had an increased objective response rate and overall survival.4 Another study directly compared cisplatin/etoposide to cyclophosphamide/epirubicin/vincristine and found the doublet improved 2- and 5-year survival rates in limited-stage SCLC.5 Carboplatin is often substituted for cisplatin due to a better toxicity profile, but some wonder if it is as efficacious as cisplatin for SCLC. A study in 1994 randomized patients to receive cisplatin/etoposide and carboplatin/etoposide for 6 total cycles. Limited-stage patients who had response to treatment and extensive-stage patients who had complete response also received thoracic radiation and prophylactic cranial irradiation with the third cycle. The cisplatin group had more adverse effects, including nausea, vomiting, neutropenic infections, and neurotoxicity, but the median survivals for the cisplatin versus carboplatin groups were 12.5 and 11.8 months, respectively. The complete response rates were also similar at 57% for the cisplatin group and 58% for the carboplatin group.6 A more recent study using the Surveillance, Epidemiology, and End Results (SEER)–Medicare database looked at whether patients 67 years and older with extensive-stage SCLC had a non-inferior survival with carboplatin/etoposide compared to cisplatin/etoposide. The results were reassuring, with both groups having nearly identical survival (35.7 weeks for cisplatin/etoposide vs. 35.9 weeks for carboplatin/etoposide). The carboplatin group also had fewer hospitalizations, emergency department visits, and intensive care unit stays. To further drive the point home, there was a meta-analysis consisting of 4 trials comparing cisplatin versus carboplatin, each combined with etoposide. The median overall survival was 9.6 months for the cisplatin patients and 9.4 months for the carboplatin patients (hazard ratio [HR] 1.08, 95% CI 0.92-1.27, p = .37). The objective response rate was 67.1% for the cisplatin group and 66% for the carboplatin group. Median progression-free survival was also similar at 5.5 months for the cisplatin group and 5.3 months for the carboplatin group.7 The main differences between these groups were the adverse effects. Carboplatin had greater hematologic toxicities, while cisplatin had more non-hematologic toxicities. Cisplatin side effects include, but are not limited to, nephrotoxicity, neurotoxicity (mainly as peripheral neuropathy), ototoxicity, nausea, vomiting, myelosuppression, metallic taste, transient elevations in liver enzymes and bilirubin, and alopecia. Myelosuppression is often a dose-limiting factor when treating SCLC patients, but growth factors are not recommended for routine use. A meta-analysis looked at a variety of chemotherapy regimens involving higher doses and accelerated schedules. Given the anticipated worsened myelosuppression with more intensive chemotherapy, these patients received growth factor, but the study found that growth factor actually had a detrimental effect on the response rate in patients on maintenance high-dose chemotherapy with no improvement in survival. Growth factor also did not have an impact on response rate in the accelerated group, and these patients actually had reduced survival. These findings were most likely due to the intensity of chemotherapy itself, but the addition of growth factor had no effect.8 Investigators have looked at combinations other than platinum/etoposide for SCLC. Adding irinotecan to cisplatin was briefly thought to be better. A Japanese trial looked at extensive-stage SCLC patients who were treated with cisplatin/irinotecan and found these patients had a median survival of 12.8 months compared to 9.4 months in those treated with the traditional cisplatin/etoposide. The 2-year survival in these patients was also an impressive 19.5% compared to 5.2% in the cisplatin group.9 The results from this study were very promising. However, they were unable to be replicated in the United States, where 2 follow-up trials failed to show improved response rate and survival with irinotecan.10,11 Some studies did find success when comparing irinotecan plus a platinum drug compared with etoposide and cisplatin or carboplatin.12,13 However, the absolute survival benefits were also met with more toxicities, so guidelines do list carboplatin or cisplatin with irinotecan as an option but only for extensive-stage disease. The recommended regimen still remains cisplatin or carboplatin in combination with etoposide. Additional studies looking at other combinations were less successful. Three-drug regimens such as cisplatin or carboplatin with etoposide and paclitaxel led to unacceptable toxicities.14 Ifosfamide with etoposide and epirubicin also increased side effects.15 Dose intensification in the treatment of SCLC remains controversial. Some studies found a modest improvement in median survival times with higher doses of chemotherapy, but many others did not.16 One study randomized untreated patients with extensive-stage SCLC to standard dose versus higher dose cisplatin/etoposide. The standard dose was defined as etoposide 80 mg/m2 on days 1 to 3 and cisplatin 80 mg/m2 on day 1 every 3 weeks. The high dose was etoposide 80 mg/m2 on days 1 to 5 and cisplatin 27 mg/m2 on days 1 to 5 every 3 weeks. Overall, there were no significant differences in median survival and complete response rates. Furthermore, patients who received higher dose chemotherapy had more toxicities, including leukopenia, thrombocytopenia, febrile neutropenia, and weight loss. Initial treatment is recommended for a total of 4-6 cycles, but additional chemotherapy beyond (eg, for maintenance) that does not appear to significantly prolong survival. A study treating extensive-stage SCLC patients with 4 cycles of cisplatin/etoposide then randomized those with stable or responding disease to observation or 4 additional cycles of treatment with topotecan. Progression-free survival appeared to be better with topotecan, but there was no difference in overall survival. There was also no difference in quality of life between those who received additional treatment versus observation.17 A meta-analysis involving 14 trials also sought to evaluate maintenance therapy versus observation in SCLC patients. The investigators found that compared to observation, maintenance therapy had no effect on 1-year mortality, 2-year mortality, overall survival, or progression-free survival.18 Subgroup analysis did show that maintenance chemotherapy improved progression-free survival in the extensive stage, but this needs to be weighed with its lack of impact on overall survival and greater risk of cumulative toxicity. The exception to maintenance therapy is with extensive-stage patients. A recent trial found that initial treatment with carboplatin/etoposide/atezolizumab followed by maintenance atezolizumab significantly improved progression-free survival and overall survival.19 Immunotherapy, however, does not currently have a role in locally advanced SCLC. For patients with locally advanced disease who are receiving systemic therapy alone, response should be evaluated after every 2 cycles of treatment using CT with contrast of the chest, abdomen, and pelvis. Disease progression during initial treatment is referred to as refractory disease (Table 17-1). Disease progression within 3 months after completion of initial treatment is refractory disease. In these cases, response to subsequent treatment is expected to be poor (<10%). Response rates are slightly better if there is disease progression more than 3 months after completing therapy. This is referred to as sensitive relapse. TABLE 17-1 Definitions of Disease Progression in Small Cell Lung Cancer Small cell lung cancer has a very high propensity to metastasize to the brain. Unlike the conflicting data regarding whether patients with extensive-stage disease should have prophylactic cranial irradiation (PCI), the data for limited-stage disease patients are reassuring. A study looking at limited-stage patients who had response to chemoradiation found better median overall survival with PCI compared to observation (26 vs. 14 months, respectively).20 A meta-analysis showed that PCI may prevent the incidence of brain metastases. The 3-year incidence of brain metastases was 33.3% in the PCI group versus 58.6% in the control group. They also had better 3-year overall survival (20.7%) compared to that in the control (15.3%).21 One other study showed patients who received PCI had better survival at 2, 5, and 10 years compared to those who did not.22 However, PCI does have drawbacks, with the main one being late neurologic complications. Patients over the age of 60 seem to have more chronic neurotoxicity, though effects may be less when PCI is given at a lower dose after completing chemotherapy. Prophylactic cranial irradiation may cause neurologic complications, especially in elderly patients. Despite being initially responsive to treatment, SCLC will likely relapse with resistant disease. Subsequent treatment is more for palliation of symptoms since the median survival with additional therapy is less than a year. If the relapse occurs more than 6 months from the completion of initial treatment, rechallenging the patient with the initial regimen is reasonable. The exception to this is extensive-stage patients initially treated with carboplatin/etoposide/atezolizumab, who then progress while on maintenance atezolizumab even if the relapse occurs more than 6 months after completing initial therapy. If relapse occurs after 6 months from completion of initial treatment, consider re-treating with the original regimen. When relapse occurs less than 6 months from initial treatment, options include a clinical trial or another second-line agent. These include the following: • Second-line agents As with many other malignancies, SCLC has also been evaluated in relation to immunotherapy. CheckMate 032 evaluated nivolumab versus various doses of nivolumab plus ipilimumab in patients with relapsed SCLC and found that 1-year overall survival was 42% in patients treated with the nivolumab/ipilimumab combination and 30% in patients who received nivolumab alone.23 CheckMate 331 compared nivolumab to topotecan or amrubicin and found similar overall survival but fewer adverse effects in the nivolumab group.24 Guidelines recommend using either nivolumab alone or with ipilimumab, though adding the second immunotherapy agent also increases side effects. Pembrolizumab has also been added to guidelines as a viable second-line option after it showed a response rate of 19.3% and a median overall survival of 7.7 months. It is important to note that both endpoints were higher in patients positive for PD-L1 (programmed death ligand 1), whereas the responses with nivolumab with or without ipilimumab were seen regardless of PD-L1 expression. If a patient progresses on one of these agents, switching to another checkpoint inhibitor is not recommended. Also, though this is not applicable to locally advanced SCLC, patients who progress on atezolizumab should not be switched to another immunotherapy agent. Small cell lung cancer is most frequently diagnosed in the elderly, who are unfortunately underrepresented in clinical studies. Many clinicians shy away from aggressively treating the elderly, though performance status is a much better predictor of response to therapy. A large study evaluated chemoradiation in elderly patients with limited-stage SCLC who received chemoradiation versus chemotherapy alone and found that patients who had the addition of radiation had a survival benefit.25 The CONVERT trial was a phase III, randomized superiority trial comparing elderly patients, aged 70 and older with younger patients in regard to concurrent once-daily versus twice-daily radiation. The study found that the elderly patients had comparable survival and toxicity.26 After response to initial therapy, however, the risks and benefits of PCI should be discussed with patients 60 years or older since they have an increased risk for cognitive decline compared to younger patients. A 65-year-old male had a 4-cm lung mass observed on PET biopsied by core needle. He smoked 2 packs of cigarettes per day and COPD and HTN. The PET revealed positive mediastinal lymph nodes. The brain MRI is normal. The pathology shows SCLC. His performance status is good, and his basic laboratory tests are normal. Learning Objectives: 1. How is limited stage defined? 2. What is the best radiation regimen? 3. What is the benefit of PCI? Prior to more sophisticated staging classification, SCLC was considered to be either limited stage or extensive stage. Criteria for limited-stage SCLC has been defined as disease that is limited to the hemithorax, mediastinum, and supraclavicular lymph nodes, which can be encompassed within a tolerable radiation field. Despite the development of TNM classification, SCLC continues to be discussed based on limited versus early stage, which, at this time, appears to be the most important tumor-related prognostic factor. Currently, only one-third of new cases are considered limited-stage disease at diagnosis, which generally correlates with the most recent AJCC eighth edition stages I, II, or III.27 Although surgery was initially thought to be a curative solution to limited-stage disease, the current treatment paradigm for these early stage patients is systemic chemotherapy with radiation to the disease in the chest after 2 studies that surgery did not improve outcomes when compared to non-invasive treatment.28,29 SCLC is a highly aggressive disease and is most often considered inoperable at the time of diagnosis. Due to the rapid proliferative rate, SCLC was found to be exquisitely sensitive to chemotherapy, and thus this became the mainstay of treatment until the 1980s, when it became clear that the addition of radiation therapy could dramatically improve outcomes. While trials supported the addition of thoracic radiation to chemotherapy in limited-stage SCLC, the most definitive evidence came from Cancer and Leukemia Group B (CALGB) 8083. Almost 400 patients with limited-stage SCLC were randomized to receive radiotherapy with chemotherapy, delayed radiotherapy plus chemotherapy, or chemotherapy alone. Overall survival, progression-free survival, and local control were all improved in the arms that received radiotherapy, at the cost of somewhat increased toxicity.30 Two meta-analyses including thousands of patients treated in over a dozen trials later published their findings that combined modality therapy including chemotherapy and radiation was superior to chemotherapy alone in limited-stage disease, with improved local control and an absolute survival benefit of 5.4% at 2-3 years after treatment. Still, the optimal volume, dose, fractionation, and sequencing of thoracic radiation remained to be elucidated.31 As mentioned, the definition of limited-stage SCLC was historically based on the ability to cover the disease within a tolerable radiation field, namely, a single radiation port, which typically included the primary tumor as well as regional and ipsilateral supraclavicular lymph nodes if they could be encompassed safely. This definition relied on older radiation therapy techniques that limited the ability to encompass disease more distant from the tumor without a corresponding increase in toxicity. With the more sophisticated techniques that are now available, the definition of limited-stage SCLC has evolved. Many practitioners now even consider some patients with contralateral mediastinal or supraclavicular lymph nodes to have limited-stage SCLC.32 Treatment of the primary tumor, the ipsilateral hilum, and the bilateral mediastinum has now been replaced with volumes that only include the primary tumor and involved nodal stations based on PET imaging. While elective nodal coverage was often performed including the ipsilateral hilum, mediastinum, and often the supraclavicular nodal regions, there is now evidence that selective nodal radiation based on PET positivity has a very low rate, less than 5%, of isolated nodal failure.33 For patients who receive chemotherapy prior to treatment, the target volume includes disease from the most current scan as well as the prechemotherapy originally involved lymph node regions based on prechemotherapy PET imaging. An internal target volume is created to encompass the disease as it moves throughout the respiratory cycle, with additional margin added for setup uncertainties. While a 3-dimensional (3-D) conformal radiation delivery technique can be used, more often physicians are choosing intensity-modulated radiation therapy (IMRT) to reduce the dose to surrounding normal structures, with a resultant decrease in toxicity. While the large majority of lung cancer histologies respond well to conventionally fractionated radiation therapy (eg, 2 Gy per day over the course of several weeks to a total dose of 60-70 Gy), SCLC was found to be exquisitely radiosensitive based on a number of radiobiologic experiments that led to the choice of a lower total dose, hyperfractionated regimen in a large Intergroup (INT) trial. INT 0096 randomized patients to 45 Gy at 180 cGy per fraction delivered daily versus a hyperfractionated regimen delivering 45 Gy at 150 cGy per fraction twice daily. The hyperfractionated treatment arm showed an overall survival benefit, albeit at the cost of increased esophagitis. The 2- and 5-year overall survival in the hyperfractionated arm was 47% and 26% versus 41% and 16% in the daily treatment arm, respectively.34 There has been some criticism of this randomization as the 45-Gy dose delivered twice daily likely has a higher biologically effective dose (BED), making it difficult to discern whether the improvement was a result of purely the fractionation schedule. A recent trial attempting to compare more biologically equivalent doses to randomized patients to either a twice-daily treatment to 45 Gy or a once-daily treatment to 66 Gy. At a median follow-up of 45 months, the median overall survival was 30 months in the twice-daily group versus 25 months in the once-daily group (p = .14) and the 2-year overall survival was 56% versus 51% in the twice- versus once-daily groups, respectively, which did not meet the 12% threshold required to deem twice-daily superior to daily treatment. This trial was not powered for equivalence; thus, similar efficacy of the two treatments cannot be included. Somewhat surprisingly, the toxicities of both fractionation regimens were relatively comparable, with no significant difference in the number of patients who developed grade 2 esophagitis (18% vs. 19%) in the twice- versus once-daily treatment arms, respectively, which is lower than seen in the twice-daily treatment arm on INT 0096 of 32%. This is speculated to stem from improved radiation treatment delivery as technology advanced between the two trials, which should support the decision to recommend hyperfractionation even in patients not in the best performance category.26 Authors from this trial’s publication do state that the twice-daily treatment regimen may have several advantages over once-daily treatment; these include improved delivery of radiation treatment that was reported on trial, as well as the ability to halve the total treatment time. More recent trials have determined that dose escalation is feasible, and we are currently awaiting results of several trials, including Radiation Therapy Oncology Group (RTOG) 0538, which is directly conventionally fractionated radiation to a total dose of 70 Gy to hyperfractionated treatment to 45 Gy.35,36
17
SMALL CELL LUNG CANCER TREATMENT: LOCALLY ADVANCED
CHEMOTHERAPY FOR LOCALLY ADVANCED SMALL CELL LUNG CANCER
CLASSIFICATION
PERFORMANCE STATUS
CHEMOTHERAPY REGIMENS
CISPLATIN VERSUS CARBOPLATIN
GROWTH FACTOR
OTHER TREATMENT OPTIONS
DOSE INTENSIFICATION
MAINTENANCE CHEMOTHERAPY
RESPONSE ASSESSMENT

PROPHYLACTIC CRANIAL IRRADIATION
SUBSEQUENT THERAPY
 Nivolumab with or without ipilimumab
Nivolumab with or without ipilimumab
 Pembrolizumab
Pembrolizumab
 Topotecan
Topotecan
 Irinotecan
Irinotecan
 Paclitaxel
Paclitaxel
 Docetaxel
Docetaxel
 Temozolomide
Temozolomide
 Vinorelbine
Vinorelbine
 Oral etoposide
Oral etoposide
 Gemcitabine
Gemcitabine
 Cyclophosphamide/doxorubicin/vincristine
Cyclophosphamide/doxorubicin/vincristine
 Bendamustine
Bendamustine
ELDERLY
RADIATION THERAPY FOR LOCALLY ADVANCED SMALL CELL LUNG CANCER
DEFINITION OF LIMITED STAGE
TREATMENT VOLUME
DOSE AND FRACTIONATION
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree