Small Cell Lung Cancer
EPIDEMIOLOGY
Lung cancer is the leading cause of cancer-related mortality in the United States with over 172,000 new cases and over 163,000 deaths in 2005 with over 225,000 new cases and 160,000 deaths in 2013. Approximately 14% of lung cancers diagnosed between 2005 and 2009 were small cell lung cancer (SCLC), with the remainder being various subtypes of non-small cell lung cancer (NSCLC) such as adenocarcinoma, squamous cell, and large cell, among others. The proportion of new lung cancers diagnosed that are SCLC has been declining over the past few decades. The reasons for this are unclear but may relate at least in part to the changing composition of cigarettes and inhalation patterns.
SCLC is associated with cigarette smoking in the vast majority of cases. Both duration of smoking and number of cigarettes per day are directly correlated with lung cancer risk. Patients who quit smoking decrease their lung cancer risk, although not to never-smoking levels (1).
PATHOLOGY
SCLC is a type of high-grade neuroendocrine lung cancer. The neuroendocrine lung tumors encompass a diverse spectrum that ranges widely in prognosis, from low-grade typical carcinoids and intermediate-grade atypical carcinoids to the higher-grade cancers including large cell neuroendocrine cancer and SCLC. SCLC and large cell neuroendocrine cancer behave similarly and have similar prognoses.
Pathologically, SCLC is defined as “a proliferation of small cells (<4 lymphocytes in diameter) with unique and strict morphologic features, scant cytoplasm, ill-defined borders, finely granular salt and pepper chromatin, absent or inconspicuous nucleoli, frequent nuclear molding, and a high mitotic count” (2). Immunohistochemical staining is generally positive for epithelial cell markers such as keratin and epithelial membrane antigen. In addition, neuroendocrine markers such as chromogranin A and synaptophysin are positive in the majority of SCLCs.
CLINICAL PRESENTATION
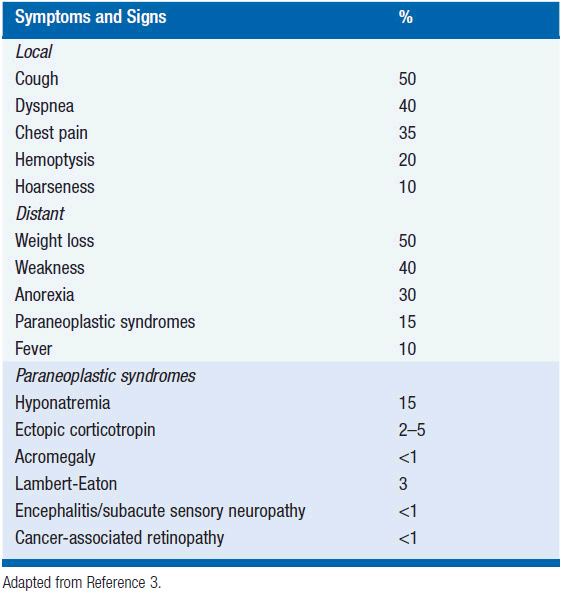
Most patients present with symptoms that are related to the intrathoracic bulk of disease or widespread dissemination. Cough, dyspnea, weight loss, and weakness are the most common presenting symptoms (3).
In addition, a variety of paraneoplastic syndromes are observed with SCLC. The ectopic production of hormones is a common culprit for the endocrine paraneoplastic disorders, which include hyponatremia (due to ectopic production of antidiuretic hormone), Cushing’s syndrome (ectopic corticotropin production), and acromegaly (ectopic growth hormone releasing hormone). Neurologic paraneoplastic syndromes such as Lambert-Eaton myasthenic syndrome are caused by autoantibody-mediated damage to the nervous system. Treatment of the underlying tumor can help control these paraneoplastic syndromes; in addition, medical management of symptoms may be indicated (see Table 52-1).
DIAGNOSIS AND STAGING
Radiographically, SCLCs tend to present as central hilar masses with bulky mediastinal lymphadenopathy. Given the central location of most SCLCs, the diagnosis is usually made by pathologic analysis of a bronchoscopic or endoscopic biopsy sample. Alternatively, percutaneous CT-guided biopsies provide another means of obtaining tissue for diagnosis.
Once the diagnosis of SCLC is made, accurate staging is important for treatment planning. SCLC is traditionally categorized into either limited stage or extensive stage disease, as described by the Veteran’s Administration Lung Group. Limited stage is typically defined as disease that involves one hemithorax or disease that is encompassable within one radiotherapy port; using the TNM staging system, this corresponds to T any, N any, M0, excluding T3-4 due to multiple nodules that do not fit within a tolerable radiation field. Extensive stage is any disease that extends beyond these parameters. The majority of patients present with extensive stage disease.
The staging workup is designed to establish whether a patient has meta-static disease, since this will substantially alter both the prognosis and treatment plan. The workup should include a CT of the chest with extension into the abdomen to evaluate the liver and adrenals, which are common sites of metastasis. Brain imaging should be performed since the CNS is a frequent site of spread. Both head CT and brain MRI are commonly used, but MRI is preferred for its greater sensitivity for detecting metastatic disease. Bone scan should be performed to assess for bony metastases. PET scans are increasingly being used for staging purposes, but its exact role is not yet clearly defined.
TREATMENT FOR LIMITED STAGE SCLC
Limited stage SCLC is treated with a combination of chemotherapy and radiation, which typically yields a response rate of greater than 80% (complete response 40%–60%) and median survival of 14–20 months. Surgery is not typically attempted due to the high early dissemination rate and poor overall survival at 5 years. Occasionally, patients present with a T1-2N0M0 cancer that is surgically resected on the presumption of NSCLC. These patients still need adjuvant chemotherapy after resection with appropriate SCLC regimens.
Combination chemotherapy and radiation remain the standard of care for limited stage SCLC. In terms of chemotherapy, cisplatin and etoposide is the standard regimen used. A randomized phase III trial demonstrated better survival among limited stage SCLC patients treated with cisplatin and etoposide in combination with radiation compared with the regimen of cyclophosphamide, epirubicin, and vincristine with radiation (4). As the cisplatin/etoposide regimen is easily combined with radiation, with little mucosal toxicity and less hematologic toxicity than other regimens, it remains the standard of care.
The addition of radiation to chemotherapy improves survival, and approximately 5% more patients are alive at 2 and 3 years when treated with combination chemoradiation versus chemotherapy alone (5). The early incorporation of radiation appears to yield better results than waiting until later in the treatment course. Several trials have addressed the timing of thoracic radiation. The National Cancer Institute of Canada randomized 308 patients receiving chemotherapy to early thoracic radiation (starting with the second cycle of chemotherapy) versus late thoracic radiation (starting with the sixth cycle). There was no difference in the total cumulative chemotherapy dose delivered between the two arms, but both progression-free survival and overall survival were improved in the early radiation arm (6). Multiple meta-analyses have also examined the benefits of early versus late thoracic radiation, and most favor early radiation (7–12). There is a suggestion that completing radiation within 30 days of starting any therapy, without compromising chemotherapy dosing, may be most beneficial.
Hyperfractionated radiation also appears to improve outcomes. Turrisi et al. randomized 417 patients with limited SCLC to cisplatin/etoposide given with either daily (qd) radiation (45 Gy total, 1.8 Gy fractions) or twice-daily (bid) radiation (45 Gy total, 1.5 Gy fractions). Patients on the bid dose schedule had better median survival (23 months for bid, vs. 19 months for qd) and 5-year survival (26% vs. 16%). Significantly worse toxicities were noted with the bid regimen, including grade III esophagitis, which occurred in 27% of this population versus 11% of the qd dosed population (13).
Not all centers have adopted the hyperfractionated dosing, however, and the question of whether hyperfractionation or total dose of radiation is more important remains debated. Choi et al. tested escalating radiation doses in both the qd and bid schedules. The bid total dose was limited to 55 Gy while the qd dose went up to 70 Gy. In long-term follow-up, both median survival (24 months vs. 29.8 months) and 5-year survival (20% vs. 36%) favored qd dosing (14).
Current NCCN guidelines recommend that radiation be delivered concurrently with chemotherapy in limited stage SCLC, starting within the first two cycles of chemotherapy. Either twice-daily dosing of radiation (to a total dose of 45 Gy in 1.5 Gy fractions) or once-daily dosing (to a total dose of 60-70 Gy) is acceptable (15).
PROPHYLACTIC CRANIAL IRRADIATION
Prophylactic cranial irradiation (PCI) should be considered in both limited and extensive stage disease where there has been response.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree