I. EPIDEMIOLOGY AND ETIOLOGY
A. Incidence. Malignant melanoma is the sixth most common cancer among women and the fifth most common cancer among men in the United States. Malignant melanoma accounts for about 4% of all skin cancers, but it is responsible for 80% of deaths. The incidence of melanoma in the United States is 18.2 cases per 100,000/year, two-thirds in males and one-third in women. The median age at the time of diagnosis is 58 years, and only 0.9% of cases are diagnosed before the age of 20. The estimated number of new cases in the United States in 2010 is 68,130, and 8,700 patients would die of melanoma.
The incidence of melanoma rose rapidly in the 1970s at about 6% per year; this rate decreased in the 1980s to about 3% per year. In the past decade, the death rate from melanoma has decreased. White people have a 17 to 25 times higher risk for the development of melanoma than blacks, but melanoma is diagnosed among all races.
B. Risk factors. The strongest risk factors for melanoma are a family history of melanoma, multiple benign or atypical nevi, and a previous melanoma. The list of additional risk factors includes immunosuppression, sun sensitivity, and exposure to ultraviolet (UV) radiation.
1. Familial factors. Approximately 10% of melanomas are familial. The higher risk of melanoma in these families may be attributed to both shared susceptibility genes and shared environment.
a. High-penetrance susceptibility genes. Two genes, CDKN2A and CDK4, are associated with high-penetrance susceptibility. Mutated CDKN2A is the most prevalent gene in families with melanoma. It is located on chromosome 9p21 and it encodes cyclin-dependent kinase inhibitor 2A (p16INK4a). CDK4 encodes cyclin-dependent kinase 4, which is one of the binding partners of p16INK4a. Mutations in CDK4 are found much less frequently than in CDKN2A. Also evidence exists for another, as yet unidentified, high-penetrance susceptibility gene at chromosome 1p22.
The higher the number of family members with melanoma, the higher the probability of carrying a high-penetrance gene. Mutated CDKN2A was found in 14% families with 2 cases of melanoma, in 67% families with 6 to 7 cases, and 100% families with 7 to 10 cases. Overall, between 20% (in Australia) and 57% (in Europe) of the cases of familial melanoma are associated with CDKN2A.
b. Low-penetrance susceptibility genes. Epidemiologic studies suggest that low-penetrance susceptibility genes are found frequently among families with melanoma. The MC1R gene, which encodes the melanocytestimulating hormone receptor, has been characterized best.
c. Familial atypical multiple mole-melanoma (FAMMM) syndrome, also known as the familial dysplastic nevus syndrome, was described in 1978
in families whose members suffered from melanoma and had multiple (usually >100) large moles of variable size and color (reddish brown to bright red) with pigmentary leakage. The median age of the development of melanoma in persons with this syndrome is 33 years, and 9% of affected people develop it before the age of 20. The syndrome is transmitted in autosomal dominant fashion, but there are sex differences in penetrance. Males develop melanoma less frequently and at an older age than women.
2. Nevi. Typical nevi are frequently precursors of melanoma, but more importantly they are markers of increased risk. One study showed that men who have at least 17 and women who have at least 12 small moles on the back are at 4.6 and 5.1 times higher risk of the development of melanoma, respectively. The lifetime risk for any mole to transform into melanoma by age 80 years is 0.03% for men and 0.009% for women.
Congenital nevi are benign neoplasms that are present at birth and composed of nevomelanocytes. The malignant potential of giant congenital nevi varies between different types. Pigmented giant nevi have especially high risk for malignant transformation. Nevus sebaceous is associated with the development of basal cell carcinoma. Verrucous epidermal nevi and woolly hair nevi do not have malignant potential.
3. Previous melanoma. The rate of a second primary cutaneous melanoma is more than 10 times higher than the first one. The 1-, 5-, and 10-year probability of developing the second primary melanoma are 1% to 2%, 2.1% to 3.4%, and 3.2% to 5.3%, respectively. The greatest risk is within the first 2 years, but it remains elevated for at least 20 years. Males, elderly patients, and individuals with the first melanoma on the face, the neck, and the trunk are at especially high risk. The incidence of a third primary melanoma from the time of second primary melanoma is 16% at 1 year and 31% at 5 years.
4. Immunosuppression. Among organ allograft recipients, melanoma constituted 5% of skin cancers and was significantly higher than in the general population (2.7%). A six- to eightfold increase in melanoma rates has been observed among male kidney transplant recipients.
5. Sun sensitivity. Light-skinned and redheaded people frequently carry a polymorphism in the melanocortin receptor gene (MC1R) that results in a decreased melanin production after exposure to ultraviolet (UV) radiation and in an increased risk of melanoma.
6. Exposure to sun and to UV radiation. It is known that UV radiation causes genetic changes in the skin, impairs cutaneous immune function, increases the local production of growth factors, and induces the formation of DNA-damaging reactive oxygen species that affect keratinocytes and melanocytes. Epidemiologic studies revealed that intermittent sun exposure and frequent sunburns, especially during childhood, increase the risk of melanoma. Chronic low-grade sun exposure may be protective, although data also show that higher total exposure to the sun is associated with a higher risk of melanoma among non-Hispanic white individuals. In addition, exposure to UV light from recreational tanning salons is emerging as an important risk factor for melanoma.
7. Occupational exposure. Exposure to coal tar, pitch, creosote, arsenic compounds, or radium increases the risk of melanoma development.
III. PATHOLOGY AND NATURAL HISTORY
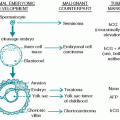
A. Pathology. Melanoma originates from melanocytes, the neural crest-derived cells that migrate into the epidermis during embryogenesis to reside in the basal layer of the epidermis. The overwhelming majority of melanomas originate in the skin, but some melanomas may arise from other primary sites. Potential extracutaneous sites include the choroidal layer of the eye and mucosal surfaces in the upper respiratory tract (most frequently, the nose and nasopharynx), gastrointestinal (GI) tract (most frequently, the anus), and genitourinary tract (most frequently, the vagina).
Several steps occur in the process of their malignant transformation. According to the Clark model, initially normal melanocytes proliferate and form a benign nevus. In the next phase, abnormal growth appears in the form of a dysplastic nevus. Melanoma may originate from a benign nevus, but it can also start from scattered melanocytes present in the normal skin. Next, in the radial growth phase, the cells acquire the ability to grow intraepidermally and have all the features of cancerous cells. Then, the lesion invades the dermis (verticalgrowth phase) and finally spreads to other organs and other areas of the skin (metastasis). Not all melanomas pass through each of these individual phases, however.
B. Molecular events in the pathogenesis of melanoma. Several molecular alterations have known pathogenic effects in the transformation of melanocytes and the evolution of melanoma. In general, germline or somatic alterations in cell-cycle control, together with somatic activating mutations or amplifications of factors involved in signal transduction pathways, are required for melanoma development.
1. Alterations in signal transduction pathways. Mutually exclusive somatic activating point mutations in NRAS (15% of melanomas) and BRAF (50% to 60% of melanomas), two members of the mitogen-activated protein kinase (MAPK) that provides proliferation signaling from surface receptors to the nucleus, are present in most melanomas of skin primary. Paradoxically, BRAF mutation is also frequent in benign nevi, where its transforming effect may be counteracted by the phenomenon of oncogene-induced senescence.
Somatic alterations in another signaling pathway important in cell growth, the phosphoinositide-3-OH kinase (PI3K) pathway, are also frequently found in melanoma, including loss of PTEN (phosphate and tensin homologue) and overexpression of protein kinase B (PKB, also known as Akt). About 20% to 40% of melanomas originating from mucosal membranes or from chronic sun damage skin and acral melanomas can harbor an activating mutation in c-kit kinase. This mutation is not found in melanoma originating from the trunk.
Finally, about 50% of uveal melanomas have an activating mutation in GNAQ or GNA11, two small GTP-binding proteins that couple cell-surface G-coupled receptors and are involved in their signal transduction.
2. Aberrant cell cycle control. As described above, inherited mutations in the CDKN2A and CDK4 genes are associated with high-penetrance susceptibility to melanoma. Somatic mutations in these and other cell-cycle control genes seem a requisite for the development of melanoma and escape from oncogene-induced senescence.
3. Other genetic events in melanoma pathogenesis. The microphthalmiaassociated transcription factor (MITF) is required for melanocyte development.
MITF gene amplifications are noted in a small subset of melanomas,
and this gene has a complex relationship with melanoma oncogenesis. Several genetic alterations common in melanoma reduce sensitivity to apoptosis, including overexpression of Bcl-2 (B-cell leukemia/lymphoma-2), silencing of APAF-1 (apoptotic peptidase activating factor-1), and activation of NF-κB (nuclear factor kappa B).
C. Major clinical-histopathologic subtypes. Traditionally melanomas have been divided based on the histologic subtypes. Recently, it has become more important to sequence individual somatic mutations in key genes (i.e., NRAS, BRAF, c-kit, GNAQ, or GNA11) and use these findings to distinguish different melanoma subtypes. The progress in understanding the molecular biology of melanoma will soon lead to further subtyping of the disease.
1. Superficial spreading melanoma compromises about 70% of all melanomas. It is most common in middle age and develops most frequently on the upper back of both sexes and on the legs of women, but it can occur in any anatomic location. Only 25% of lesions are associated with a pre-existing nevus. It spreads laterally (radial growth) for a period of time before it becomes invasive. The lesions appear as variably pigmented plaques or macules that have a bizarre shape with irregular borders. As the lesion progresses, the shape becomes more irregular and areas of regression can be noted. Progression correlates with the evolution of multiple shades of color from red (inflammation) through gray (regressed areas) to black (neoplastic melanocytes).
2. Nodular melanoma compromises about 15% to 20% of all melanomas. It is more common among older adults (the fifth and sixth decade of life), and it occurs twice as frequently in male than in female patients. The lesion appears as a darkly pigmented dome-shaped or polypoid nodule that can ulcerate and bleed early. Occasionally, it can be amelanotic. These tumors grow rapidly and vertically from the onset.
3. Lentigo maligna melanoma (4% to 15% of melanomas) is most commonly seen in older individuals (in the sixth and seventh decade of life). It arises in sun-damaged areas of the skin, mainly on the face (90% cases). The lesion appears as a tan-brown macule, very often large in size (3 to 6 cm). The lesion grows slowly and the radial growth phase may last between 5 and 50 years before it starts growing vertically. Partial regression is not uncommon during evolution. The radial growth phase is called lentigo maligna (LM) or Hutchinson freckle.
4. Acral lentiginous melanoma is the least common variant of radial growth phase melanomas. It compromises only 2% to 8% of melanomas in whites, but 30% to 75% cases in blacks, Hispanics, and Asians. It appears on the palms, soles, and terminal phalanges as a dark brown to black, unevenly pigmented patch. Small elevation may suggest vertical growth.
5. Rare types
a. Nevoid melanoma resembles benign nevi. It has verrucoid or dome-shaped appearance and can metastasize.
b. Desmoplastic melanomas resemble a scar or fibroma and appear mainly on sun-exposed areas. Very often they are amelanotic. They tend to recur locally or as isolated metastasis.
D. Mode of spread. Melanoma first spreads through the lymphatic system forming satellite lesions and in-transit metastases and then it involves regional lymph nodes.
Satellite lesions are skin or subcutaneous lesions within 2 cm of the primary tumor and represent intralymphatic extension of the tumor.
In transit metastases are defined as lesions that are >2 cm from the primary tumor, but not beyond the regional lymph node basin. Melanoma also spreads
hematogenously, sometimes after the nodal spread or skipping the draining nodes, forming distant metastases in the skin, subcutaneous soft tissue, lungs, liver, brain, and other organs.
E. Metastatic melanoma from an unknown primary site accounts for approximately 2% to 6% of all melanoma cases. It is assumed that in most these cases the primary cutaneous melanoma regressed spontaneously. Metastases most often develop as cutaneous or subcutaneous nodules or as lymph node metastases. The survival of patients with unknown primary melanoma is similar to that of patients with known primary tumors when corresponding stages are compared.
F. Paraneoplastic syndromes. Most paraneoplastic syndromes occur in patients with widely metastatic melanoma, but some may precede the diagnosis (i.e., dermatomyositis, pemphigus, and melanosis). A variety of paraneoplastic syndromes are associated with melanoma and can affect multiple organ systems, including
1. Skin (vitiligo, pemphigus, dermatomyositis, melanosis, acanthosis nigricans, systemic sclerosis). Generalized melanosis is a syndrome of progressive grayblue skin discoloration frequently accompanied by melanuria, and sometimes also by melanoptysis or a dark brown blood serum. Melanosis is caused by the melanin (or its precursor) that is produced and secreted in an increased amount by malignant cells, and then deposited within macrophages throughout the body.
2. Eyes. Melanoma-associated retinopathy is a paraneoplastic syndrome characterized by frequent sudden onset of symptoms of night blindness, light sensations, visual loss, defect in visual fields, and reduced b-waves in the electroretinogram.
3. Blood (leukemoid reaction, eosinophilia, autoimmune neutropenia)
4. Endocrine system (hypercalcemia, Cushing syndrome, hypertrophic osteoarthropathy)
5. Central and peripheral nervous system (chronic inflammatory demyelinating polyneuropathy, opsoclonus-myoclonus)
V. STAGING SYSTEM AND PROGNOSTIC FACTORS
A. Staging system. The current staging system was developed by the American Joint Committee on Cancer (AJCC) in 2010 (
Table 16.1).
B. Prognostic factors
1. Primary lesion. Tumor thickness and ulceration are the most powerful predictors of survival.
a. Tumor thickness as a prognostic factor was first described by Alexander Breslow and it is traditionally reported as Breslow thickness in millimeters. The AJCC staging system uses 1-, 2-, and 4-mm cutoffs, but tumor thickness is really a continuous prognostic variable.
b. Ulceration (the absence of intact epithelium over the tumor determined by pathologic analysis) indicates aggressive biology of melanoma.
c. Mitotic rate. Increased mitotic rate correlates with a decreased survival.
d. Clark levels, which specify the anatomic depth of invasion (see
Fig. 16.1), are no longer utilized in the most recent staging system.
2. Status of the regional lymph nodes. The total number of nodal metastases is a significant predictor of outcome in patients with lymph node involvement. Moreover, patients in whom lymph node involvement was detected clinically have worse prognosis than those who required microscopic analysis. Satellite and in-transit lesions are considered an intralymphatic spread.
3. Metastatic disease. Patients who have nonvisceral metastases (skin, subcutaneous tissue, lymph nodes) carry a better prognosis than those who have visceral metastases. Elevated level of lactate dehydrogenase (LDH) is a poor prognostic factor.
4. Survival according to the stage