Chapter 34 Sinonasal Cancer
Although the American Joint Committee on Cancer (AJCC) divides the nasal cavity into four subsites1 (the septum, floor, lateral wall, and vestibule), the nasal vestibule is best considered independently because cancers of the nasal vestibule do not spread locally until late in their course. The nasal vestibule is the internal surface of the anterior nose and is the entrance to the nasal cavity.2 It is a hair-bearing area lined by skin containing sebaceous glands and hair follicles. The nasal vestibule is divided from the remainder of the nasal cavity by the limen nasi, a ridge that is the mucocutaneous junction, 2 cm from the vestibule entrance (Fig. 34-1). The limen nasi can be found at the junction of the lower lateral alar and upper lateral alar cartilages (see web-only Fig. 34-1 available on the Expert Consult website),![]() but differentiation of nasal vestibule cancers from cancers of the rest of the nasal cavity is rarely a problem. The paranasal sinuses (frontal, ethmoidal, sphenoidal, and maxillary) are air-filled outgrowths of the nasal cavity into the respective cranial bones. Secretions from these sinuses drain through meati of the lateral nasal walls.
but differentiation of nasal vestibule cancers from cancers of the rest of the nasal cavity is rarely a problem. The paranasal sinuses (frontal, ethmoidal, sphenoidal, and maxillary) are air-filled outgrowths of the nasal cavity into the respective cranial bones. Secretions from these sinuses drain through meati of the lateral nasal walls.
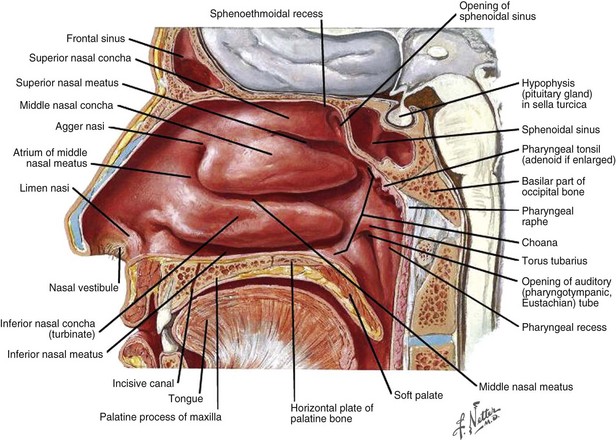
Figure 34-1 Anatomy of sinuses.
Netter Medical Illustration used with permission of Elsevier. All rights reserved.
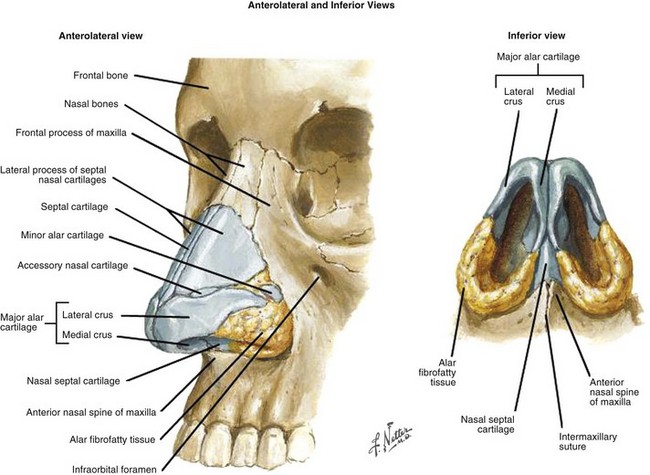
Web-Only Figure 34-1 Anatomy of sinuses. Anterolateral and inferior views.
Netter Medical Illustration used with permission of Elsevier. All rights reserved.
The treatment outcome of patients with carcinoma of the maxillary and ethmoid sinuses has improved if one compares the results by decades from the 1960s to the 1990s.3 Advances have been made in methods of extirpation, including skull base surgery, reconstruction, and highly conformal RT techniques, that enable avoidance of adjacent critical normal tissues.4 Many are gaining experience with endoscopic resection, but long-term data on efficacy of this technique as well as irradiation using protons are still lacking. The role of chemotherapy for most of these neoplasms has yet to be defined.4
Etiology and Epidemiology
Cancers of the nasal cavity and paranasal sinuses are uncommon neoplasms, with approximately 2000 Americans diagnosed per year.5 Tumors of the maxillary sinus are twice as frequent as those of the nasal cavity and ethmoid sinus, and neoplasms of the frontal and sphenoid sinuses are extremely rare. Sinonasal neoplasms occur twice as frequently in men as in women. These tumors are more frequently diagnosed in some regions outside the United States, such as Japan and South Africa.
A number of sinonasal cancers are relatively more common in people engaged in certain occupations or exposed to some chemical compounds.6–11 Excessive numbers of sinonasal squamous cell cancers were seen in bakers, pastry cooks, grain millers, construction workers, carpenters and joiners with at least 15 years in the wood manufacturing industry, farm workers, and female textile workers.12 Squamous cell carcinoma of the nasal cavity develops more often in nickel workers.13 Adenocarcinoma of the nasal cavity and ethmoid sinus occurs more frequently in carpenters and sawmill workers who are exposed to the dust of mainly hard and exotic woods.6,7,9,11 From a review of 358 sinonasal cancers with or without exposure to wood dust, TP53 gene mutations occurred in all histologic types with an overall frequency of 77% but were most common in adenocarcinoma (odds ratio [OR] 2 compared with squamous cell cancer).14 The risk of TP53 mutations was significantly increased in association with the duration and average and cumulative levels of wood dust exposure. KRAS gene mutations were reported in 13% of sinonasal adenocarcinomas.15 Synthetic wood, binding agents, and glues may also be involved as cocarcinogens.9
A pooled analysis of 12 case-controlled studies evaluated occupational exposure to formaldehyde, silica dust, textile dust, coal dust, flour dust, asbestos, and man-made vitreous fibers as risk factors for the development of sinonasal cancers.16 Pooled analysis suggested that formaldehyde was a risk factor for sinonasal adenocarcinoma development; the suggestion is tempered, however, by the fact that workers exposed to both wood dust and formaldehyde had a very strong risk and not enough workers exposed to formaldehyde only were studied to determine the significance of the formaldehyde exposure. The International Agency for Research on Cancer (IARC) found that with high professional exposure to formaldehyde and limited wood dust exposure (e.g., as may occur for embalmers, pathologists, or chemical workers), there were no increased mortality rates from sinonasal cancers.17
Maxillary sinus carcinoma was associated with a radioactive thorium-containing contrast material, thorium dioxide (Thorotrast), used for radiographic study of the maxillary sinuses decades ago.8,9 Occupational exposure during the production of chromium, mustard gas, isopropyl alcohol, and radium also increases the risk for sinonasal carcinomas.8
Cigarette smoking is reported to increase the risk of nasal cancer, with a doubling of risk among heavy or long-term smokers and a reduction in risk after long-term cessation. After adjustment for smoking, a significant dose-response relationship was also noted between alcohol drinking and risk of nasal cancer.11
Pathology, Biology, and Pathways of Spread
The clinical disease course and prognosis depend on the anatomic site, histologic type, and stage of the tumor. The size and configuration of the paranasal sinuses change with age (see web-only Fig. 34-2 available on the Expert Consult website). ![]()
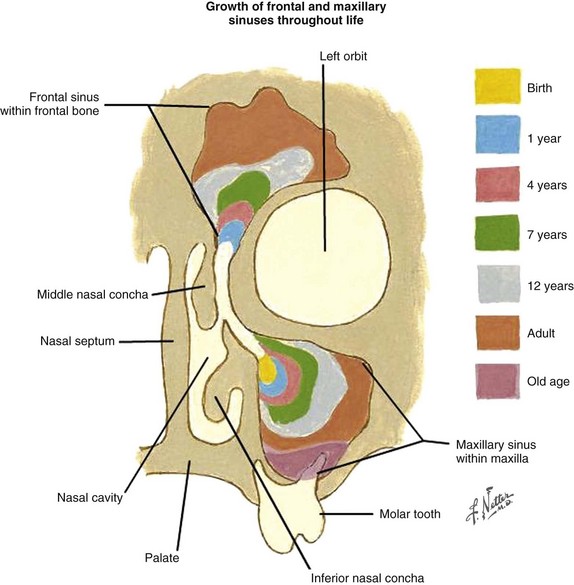
Web-Only Figure 34-2 Paranasal sinuses. The size and configuration change with age.
Netter Medical Illustration used with permission of Elsevier. All rights reserved.
Classifying cancers into the “bins” of squamous cell carcinoma (squamous cell carcinoma, transitional carcinoma, and verrucous carcinoma), adenocarcinoma, glandular carcinoma (adenoid cystic carcinoma and mucoepidermoid carcinoma), and undifferentiated carcinoma, Dulguerov and colleagues3 identified 386 patients with nasal and paranasal sinus tumors treated between 1975 and 1995 and excluded nasal vestibule cancers. Squamous cell carcinoma was the most frequent histologic type but was found in only a third of the patients (Table 34-1).
TABLE 34-1 Histologic Types of Sinonasal Cancer
| Histologic Type | No. Patients | Percentage |
|---|---|---|
| Squamous cell carcinoma | 126 | 32.6 |
| Sarcoma | 52 | 13.5 |
| Esthesioneuroblastoma | 42 | 10.9 |
| Glandular cancer | 39 | 10.1 |
| Adenoid cystic carcinoma | 35 | 9.1 |
| Lymphoma | 38 | 9.8 |
| Melanoma | 34 | 8.8 |
| Undifferentiated type | 30 | 7.8 |
| Adenocarcinoma | 25 | 6.5 |
Data from Dulguerov P, Jacobsen MS, Allal AS, et al: Nasal and paranasal sinus carcinoma. Are we making progress? A series of 220 patients and a systemic review, Cancer 92(12):3012-3020, 2001.
Nasal Vestibule
The nasal vestibules are the two entry points into the nasal cavity. Each is a triangle-shaped space situated in front of the limen nasi and defined laterally by the lateral crus and alar fibrofatty tissue and medially by the medial crus of the alar cartilage and the nasal septum, the distal end of the cartilaginous septum, and the columella (see Fig. 34-1, as well as web-only Fig. 34-1 available on the Expert Consult website). ![]() The nasal vestibule is covered by skin, and, therefore, lesions at this location are essentially squamous cell skin cancers18 but may occasionally be basal cell carcinomas,19 sebaceous carcinomas,20 melanomas,21 or non-Hodgkin’s lymphomas.22 Nasal vestibule cancers are analogous to cancers of the anal margin. Human papillomavirus has been implicated in the development of nasal septal cancers.23
The nasal vestibule is covered by skin, and, therefore, lesions at this location are essentially squamous cell skin cancers18 but may occasionally be basal cell carcinomas,19 sebaceous carcinomas,20 melanomas,21 or non-Hodgkin’s lymphomas.22 Nasal vestibule cancers are analogous to cancers of the anal margin. Human papillomavirus has been implicated in the development of nasal septal cancers.23
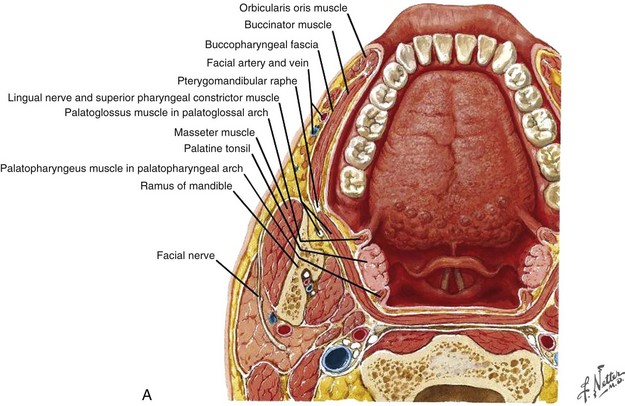
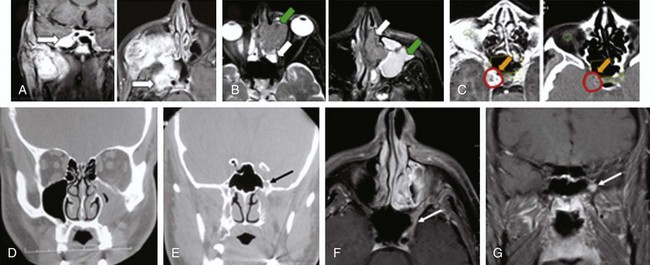
Cancers of the nasal vestibule are said to arise preferentially on the medial wall, which is the nasal septum.24 Nasal vestibule carcinomas can spread by invasion of the upper lip, gingivolabial sulcus, premaxilla, or nasal cavity. Vertical invasion may result in septal (membranous or cartilaginous) perforation or alar cartilage destruction. Lymphatic spread from nasal vestibule carcinomas is usually to the ipsilateral facial (buccinator and mandibular) and submandibular nodes. The buccinator nodes25 can be found on the buccinator muscle or in the fat of the buccinator space (Fig. 34-2, A and B).
The buccinator nodes are divided into anterior or posterior buccinator nodes depending on their relationship to the facial vein. The mandibular nodes25 include the supramaxillary, supramandibular, and inframandibular nodes and lie along the external surface of the mandible adjacent to the facial artery and anterior to the masseter muscle. Large lesions extending across the midline may spread to the contralateral facial or submandibular nodes. The average incidence of nodal metastasis at diagnosis is approximately 5%.15,16,17,18,19 Without elective lymphatic treatment, about 15% of patients develop nodal relapse.15,16,17,18 In the intensity-modulated radiation therapy (IMRT) era, when parotid sparing is de rigueur, the 15% incidence of nodal relapse makes consideration of elective nodal irradiation mandatory. Hematogenous metastases are rare in nasal vestibule carcinomas.17
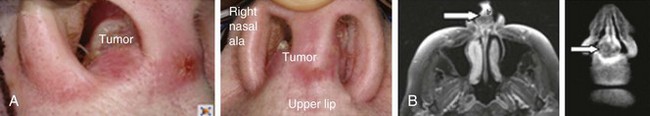
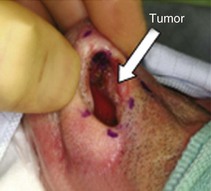
Examples of nasal vestibule tumors are shown in the photographs and MRIs in Figures 34-3A and B, and 34-4.
Nasal Cavity and Ethmoid Air Cells
Nasal cavity and ethmoid sinus tumors now share a common staging system.1 The nasal cavity proper (nasal fossa), which excludes the nasal vestibule, begins at the limen nasi anteriorly and ends at the choana posteriorly. It extends from the hard palate inferiorly to the cribriform plate superiorly. The lateral walls consist of three thin bony structures, the superior, middle, and inferior turbinates, that project inferomedially into the nasal cavity (see web-only Fig. 34-3 available on the Expert Consult website). ![]() The middle turbinate attaches superiorly to the cribriform plate and then posteriorly and laterally to the lamina papyracea.26
The middle turbinate attaches superiorly to the cribriform plate and then posteriorly and laterally to the lamina papyracea.26
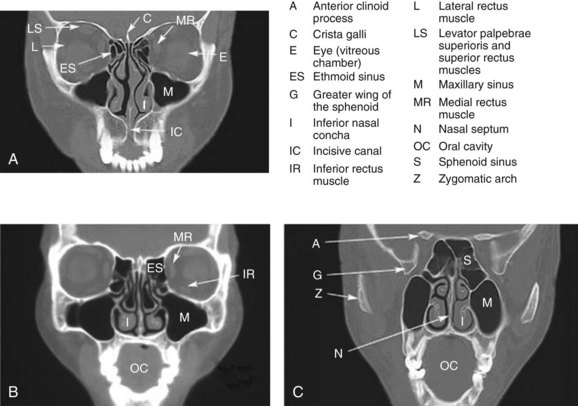
Web-Only Figure 34-3 Coronal CT images of the head from anterior (A) to posterior (C).
Netter Medical Illustration used with permission of Elsevier. All rights reserved.
The region inferior to each turbinate (or concha) is defined as the superior, middle, or inferior meatus (see web-only Fig. 34-4 ![]() available on the Expert Consult website).
available on the Expert Consult website).
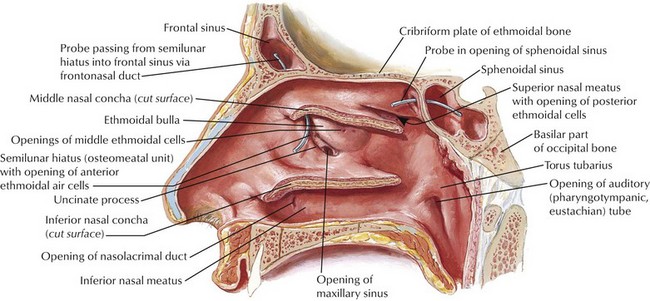
Web-Only Figure 34-4 Lateral wall of nasal cavity with nasal conchae removed.
Netter Medical Illustration used with permission of Elsevier. All rights reserved.
The superior meatus receives drainage from the posterior ethmoid air cells and the sphenoid sinus at the sphenoethmoidal recess. The middle meatus is a particularly important site of drainage, taking drainage from the anterior ethmoids, frontal sinus, and maxilla. The anterior ethmoid air cells drain into the ethmoid bulla at the superior aspect of the ostiomeatal complex, the frontal sinus drains into the middle meatus at the anterior portion of the ostiomeatal complex, and the maxillary sinus dumps its contents past the infundibulum (a valve at the superior medial aspect of the maxilla) into the middle meatus by way of the maxillary ostium at the inferior ostiomeatal complex (see red arrowhead in Fig. 34-5). Of course, the inferior meatus receives drainage from each nasolacrimal duct. (Many of these drainage petterns are illustrated in web-only Fig. 34-4![]() .)
.)
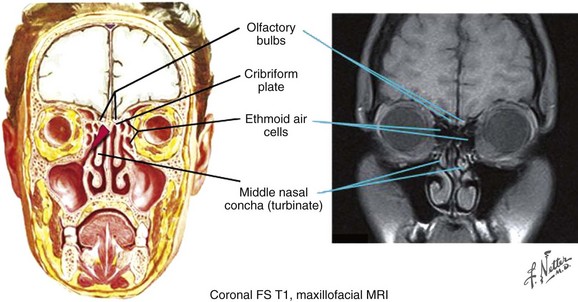
Figure 34-5 Olfactory bulbs. Coronal frontal section and maxillofacial MRI.
Netter Medical Illustration used with permission of Elsevier. All rights reserved.
The septum divides the nasal cavity into right and left halves (Fig. 34-6). The olfactory nerves penetrate the cribriform plate and innervate the roof of the nasal cavity, superior nasal turbinate, and upper third of the septum. This portion of the nasal cavity is referred to as the olfactory region. The remaining part of the cavity, the respiratory region, contains orifices connecting the nasal cavity with the paranasal sinuses.
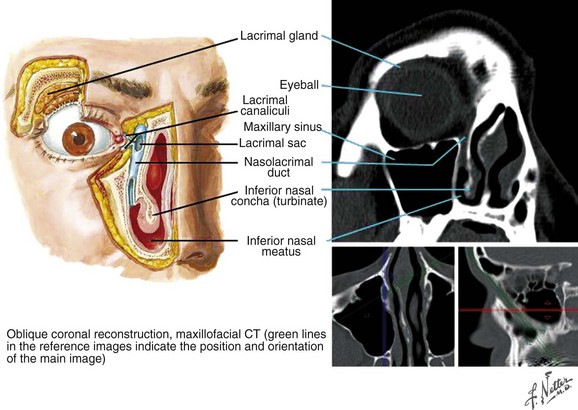
Figure 34-6 Orbit, coronal section.
Netter Medical Illustration used with permission of Elsevier. All rights reserved.
The ethmoid sinuses have several small cavities, called ethmoid air cells, within the ethmoidal labyrinth located below the anterior cranial fossa and between the nasal cavity and the orbit (see web-only Fig. 34-5 available on the Expert Consult website). ![]() They are separated from the orbital cavity by a thin, porous bone called the lamina papyracea and from the anterior cranial fossa by a portion of the frontal bone, the fovea ethmoidalis.
They are separated from the orbital cavity by a thin, porous bone called the lamina papyracea and from the anterior cranial fossa by a portion of the frontal bone, the fovea ethmoidalis.
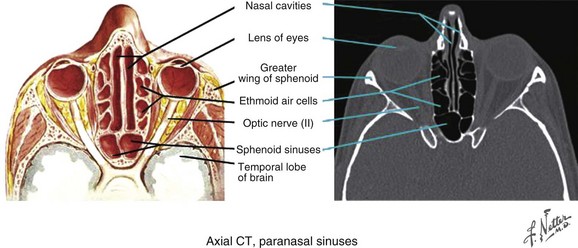
Web-Only Figure 34-5 Paranasal sinuses, axial CT scan.
Netter Medical Illustration used with permission of Elsevier. All rights reserved.
Lymphatic spread of respiratory region primary tumors is variable. Turning first to the nasal cavity, where approximately 50% of patients have a squamous cell histologic type,27 a review of 23 studies with a median follow-up of 4.4 years found the weighted average regional recurrence rate for squamous cell cancers of the nasal cavity to be 18.1%.28 At least some patients from 7 of the 23 studies received prophylactic cervical irradiation, which significantly lowered the rate of neck failure.
The review of the Instituto Nazionale per lo Studio e la Cura dei Tumori of Milan experience from 1968 to 200329 identified 305 consecutive tumors of the ethmoid sinus. There was a striking male predominance of 70.8% versus 29.2%. Intestinal-type adenocarcinoma was the most frequent histologic type (50.2%), with only 10.8% of ethmoid sinus cancer patients having squamous cell cancer. Other series disagree (see later discussion). The strong male predominance was confirmed in a 202-person study of European patients with adenocarcinomas of the ethmoid sinus.29 Only 1.6% of 305 patients with ethmoid sinus cancer presented with adenopathy, and with a median follow-up of 109 months, the cumulative incidence of lymphatic recurrence was 4.3%.29 It is important to note that most neck recurrences were accompanied by recurrence of the primary tumor. The 5-year cumulative incidence of metastases was just 3.6%.
Esthesioneuroblastoma is a tumor of neural crest origin first reported by Berger and Luc in 1924 as esthesioneuroepithelioma olfactif.30 It has been given a variety of other names, including olfactory neuroblastoma and esthesioneurocytoma. Esthesioneuroblastoma constitutes approximately 3% of all intranasal neoplasms. About 250 cases were reported in the literature between 1924 and 1990.31 Typical histologic findings in esthesioneuroblastoma are round, oval, or fusiform cells containing neurofibrils with pseudorosette formation and diffusely increased microvascularity.32 Perivascular palisading of cells can be observed in the absence of pseudorosette formation.
The route of contiguous spread of esthesioneuroblastomas is similar to that of ethmoid carcinomas. Multiple and late local and regional recurrences are the rule. Eventual development of neck disease has been reported in 21 of 110 patients33 and in 20.2% of 678 patients,34 with 61.7% of the cervical tumors occurring more than 6 months after the diagnosis.
Maxillary Sinus
Maxillary sinuses, the largest of the paranasal sinuses, are pyramid-shaped cavities located in the maxillae. The base of the maxillary sinus forms the inferior part of the lateral wall of the nasal cavity. The roof of the maxillary sinus is formed by the floor of the orbit, which contains the infraorbital canal, and the floor is composed of the alveolar process. The apex extends toward, and frequently into, the zygomatic bone. Ohngren’s line is a theoretical line passing from the medial canthus of the eye to the angle of the mandible; it is used to divide maxillary sinus cancers into suprastructure and infrastructure tumors. Tumors superior to this arbitrary plane were felt to invade vital structures such as the orbit and middle cranial fossa earlier and, therefore, have a worse prognosis.35 Also of importance when understanding the anatomy of the maxillary sinus is the pterygopalatine fossa. The pterygopalatine fossa, also known as the sphenopalatine fossa, can be thought of as a box, with its anterior wall being the posterior wall of the maxillary sinus. The posterior wall is made up of the pterygoid plates and inferior aspect of the sphenoid bone, and the roof is the inferior orbital fissure. The floor is the palatine canal and contains the greater palatine nerve. Medially it is defined by the perpendicular plane of the palatine bone. The pterygopalatine fossa contains the maxillary nerve (V2), which enters from the brain via the foramen rotundum, as well as the pteryogopalatine ganglion and the distal maxillary artery, which enters via the pterygomaxillary fissure. The pteryogopalatine ganglion is the largest head and neck parasympathetic ganglion and has sensory, motor, and sympathetic functions.
Sixty-two percent of maxillary sinus cancers are squamous cell carcinomas.3,36 Other histologic types and survival rates are shown in Table 34-2.
The pattern of spread of maxillary sinus cancer varies with the site of origin. Neoplasms of the suprastructure tend to extend into the nasal cavity, nasopharynx, ethmoid cells, inferior or medial wall of the orbit, orbital contents, pterygopalatine fossa, masticator space, infratemporal fossa, base of the skull, parasellar region and cavernous sinus, and middle cranial fossa, as shown in Figure 34-7. Tumors of the infrastructure extend to the palate, alveolar process, gingivobuccal sulcus, soft tissue of the cheek, nasal cavity, masseter muscle, pterygopalatine space, and pterygoid fossa. It is sometimes difficult to differentiate these tumors from tumors extending from the upper alveolus and hard palate.
Overall, the incidence of clinical lymphadenopathy is relatively low at diagnosis, but because regional recurrence is common, the presumption is that subclinical nodal disease exists at presentation.25 Cantu and associates29 reported that 8.3% of 399 patients presented with lymphadenopathy, and during follow-up (median, 109 months), 12.5% developed regional recurrence. Squamous cell primary cancers of the maxilla had a 10.3% incidence of nodal disease at presentation, with a 36.4% recurrence rate in node-positive patients.29 The cumulative incidence of neck recurrence for patients with squamous cell cancer of the maxilla was 20.7%.29 Le37 has published the location of nodal presentation and failure and found that levels IB, II, and V and the preauricular area can be involved ipsilaterally and level II contralaterally.
Sinonasal Undifferentiated Carcinoma
Sinonasal undifferentiated carcinoma (SNUC) has recently been identified by the World Health Organization38 as a distinct sinonasal neoplasm that should be differentiated from esthesioneuroblastoma and other cancers.39 There is a male predominance, and tumors typically are aggressive, presenting with locally extensive disease. The etiology is unknown. Immunohistochemical studies are noncontributory, and stains for epithelial mucin are negative. The nuclear to cytoplasmic ratio is high, with a very high mitotic rate. In 2005, fewer than 100 cases of SNUC had been described; by 2008, approximately 200 cases had been described40; and a review from 2009,39 as well as anecdotal experience, suggested that the diagnosis was becoming more common. Scanty evidence (17 patients) reported that SNUC is one of the few ethmoid sinus cancers with a 25.5% rate of regional failure. A 13% rate of regional failure in 26 SNUCs from the maxilla was reported.29 Mendenhall’s review of the literature41 found that 10% to 30% of SNUC patients presented with clinically positive adenopathy, and the cure rates varied from 20% to 50%.
Clinical Manifestations, Patient Evaluation, and Staging
Table 34-3 summarizes the updated American Joint Committee on Cancer (AJCC) TNM classification (seventh edition) for cancers of the maxillary sinus, ethmoid sinus, and nasal cavity.1 Significant updates in the seventh edition are as follows:
TABLE 34-3 Nasal Cavity and Paranasal Sinus Cancers: TNM Staging Classification by the American Joint Committee on Cancer
| Primary Tumor (T) | |
| Maxillary Sinus | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to maxillary sinus mucosa with no erosion or destruction of bone |
| T2 | Tumor causing bone erosion or destruction, including extension into the hard palate and/or the middle of the nasal meatus, except extension to the posterior wall of maxillary sinus and pterygoid plates |
| T3 | Tumor invades any of the following: bone of the posterior wall of maxillary sinus, subcutaneous tissues, floor or medial wall of orbit, pterygoid fossa, ethmoid sinuses |
| T4a | Moderately Advanced Local Disease Tumor invades anterior orbital contents, skin of cheek, pterygoid plates, infratemporal fossa, cribriform plate, sphenoid or frontal sinuses |
| T4b | Very Advanced Local Disease Tumor invades any of the following: orbital apex, dura, brain, middle cranial fossa, cranial nerves other than maxillary division of trigeminal nerve (V2), nasopharynx, or clivus |
| Nasal Cavity and Ethmoid Sinus | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor restricted to any one subsite, with or without bony invasion |
| T2 | Tumor invading two subsites in a single region or extending to involve an adjacent region within the nasoethmoidal complex, with or without bony invasion |
| T3 | Tumor extends to invade the medial wall or floor of the orbit, maxillary sinus, palate, or cribriform plate |
| T4a | Moderately Advanced Local Disease Tumor invades any of the following: anterior orbital contents, skin of nose or cheek, minimal extension to anterior cranial fossa, pterygoid plates, sphenoid or frontal sinuses |
| T4b | Very Advanced Local Disease Tumor invades any of the following: orbital apex, dura, brain, middle cranial fossa, cranial nerves other than (V2), nasopharynx, or clivus |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
| N2 | Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension, or in multiple ipsilateral lymph nodes, ≤6 cm in greatest dimension, or in bilateral or contralateral lymph nodes, ≤6 cm in greatest dimension |
| N2b | Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension |
| N2c | Metastasis in multiple ipsilateral lymph nodes, ≤6 cm in greatest dimension |
| N3 | Metastasis in bilateral or contralateral lymph nodes, ≤6 cm in greatest dimension Metastasis in a lymph node >6 cm in greatest dimension |
| In clinical evaluation, the actual size of the nodal mass should be measured, and allowance should be made for intervening soft tissues. Most masses >3 cm in diameter are not single nodes but confluent nodes or tumors in soft tissues of the neck. There are 3 stages of clinically positive nodes: N1, N2, and N3. The use of subgroups a, b, and c is not required but is recommended. Midline nodes are considered homolateral nodes. | |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| AJCC Stage Groupings | |
| Stage 0 | Tis N0M0 |
| Stage I | T1N0M0 |
| Stage II | T2N0M0 |
| Stage III | T3N0M0 or T1-3N1M0 |
| Stage IVA | T4aN0-1M0 or T1-4aN2M0 |
| Stage IVB | T4bN anyM0 or TanyN3M0 |
| Stage IVC | TanyNanyM1 |
From Edge SB, Byrd DR, Compton CC, et al, editors: AJCC Cancer Staging Handbook, ed 7, New York, 2010, Springer.
Nasal Vestibule
Small carcinomas of the nasal vestibule usually present as asymptomatic plaques or nodules, often with crusting and scabbing. The septum is the most common location.24,42 Advanced lesions may extend to adjacent structures, such as the upper nasal septum, upper lip, infranasal depression (philtrum), skin of the nose, nasolabial fold, hard palate, or buccogingival sulcus. Deep muscle and bone and nerve involvement may cause pain accompanied by bleeding or ulceration. Large ulcerated lesions may become infected, leading to severe tenderness that hinders complete clinical assessment. The contiguous spread is best assessed by bimanual palpation and examination through a nasal speculum or fiberoptic endoscope after mucosal decongestion and topical anesthesia. Early buccinator and submandibular nodal involvement may be detected by bimanual palpation or by imaging.25 Staging of nasal vestibule carcinoma is by the TNM classification system for the skin. Other staging systems have been used in reporting data, however.43,44
Nasal Cavity and Ethmoid Air Cells
Carcinoma
The presenting symptoms and signs of nasal fossa tumors are similar to those associated with nasal polyps: chronic unilateral nasal discharge, ulcer, obstruction, anterior headache, and intermittent nosebleeds. This similarity often leads to delays in diagnosis. Other symptoms and signs include nasal ulcer, tenderness, facial swelling, or pain.21 Invasion of the orbital cavity may produce a palpable medial orbital mass, proptosis, and diplopia; obstruction of the nasolacrimal duct causes epiphora (overflow of tears); involvement of the olfactory region may produce anosmia (inability to perceive odors) and expansion of the nasal bridge; and extension through the cribriform plate results in frontal headache.
Information on imaging studies can be found on the Expert Consult website.![]()
Primary tumors of the lateral wall and floor tend to be diagnosed in more advanced stages. Of the patients referred to the M.D. Anderson Cancer Center (MDACC) between 1969 and 1985, 3 (21%) of 14 patients with septal lesions versus 20 (65%) of 31 patients with lateral wall or floor primary tumors had disease extension beyond the nasal cavity (stages II and III).45,46
Esthesioneuroblastoma
The natural history of esthesioneuroblastoma is long and multiple recurrences are the rule. A literature review of 97 patients reported between 1969 and 1977 found that the age distribution is bimodal, with a first peak incidence occurring between 11 and 20 years and a second, higher peak incidence between 51 and 60 years.47 The Surveillance, Epidemiology, and End Results (SEER) analysis of 311 patients found the age distribution to be unimodal, with the mean age being 53 years.48 SEER analysis described the primary site as the nasal cavity in over three-fourths of cases and 12.3% presented with nodal involvement.
The most common presenting symptoms are nasal obstruction and epistaxis. Anosmia (inability to perceive odors) can precede diagnosis by many years.49 Other symptoms are related to contiguous disease extension into the orbit (proptosis, visual field defects, orbital pain, epiphora), paranasal sinuses (medial canthus mass, facial swelling), and anterior cranial fossa (headache) and to manifestation of inappropriate antidiuretic hormone secretion. Most present in the nasal cavity (web-only Fig. 34-6). ![]()
Physical evaluation and recommended staging workup procedures for esthesioneuroblastoma are similar to those for nasal fossa tumors. The commonly used staging system, proposed by Kadish and colleagues,32 is as follows:
The stage distribution of the 97 patients reviewed by Elkon and colleagues47 at diagnosis is 30% stage A, 42% stage B, and 28% stage C. Of the 22 patients who had stage C disease, 9 had orbital involvement, 6 had invasion of the cribriform plate or cranial fossa, 11 had palpable neck nodes, and only 1 had distant metastasis.
Maxillary Sinus
Because of their relatively silent presentations, maxillary cancers are often diagnosed late. Of the 73 patients referred to the MDACC for treatment between 1969 and 1985, for example, symptoms and signs related to extension to the premaxillary region (facial swelling, pain, or paresthesia of the cheek) occurred in 26 (36%). Symptoms due to tumor spread to the nasal cavity (e.g., epistaxis, nasal discharge or obstruction) or oral cavity (e.g., ill-fitting denture, alveolar or palatal mass, or unhealed tooth socket after extraction) occurred in 20 (27%) and 19 (26%), respectively. Five patients (7%) presented with symptoms and signs of orbital invasion such as proptosis, diplopia, impaired vision, or orbital pain.50
The T classification description by the AJCC staging system is shown in Table 34-3 and is based on clinical and radiologic evidence of involvement of various adjoining anatomic structures.
Primary Therapy
Nasal Vestibule
Nasal vestibule cancers are skin cancers that are in a cosmetically important location. Radiotherapy is generally preferred for carcinoma of the nasal vestibule because of better cosmetic outcome, but if local excision of a medial tumor of the nasal septum can be performed with clear margins and no cosmetic deformity, that treatment may well be more appropriate.24 Surgery can yield a high control rate with excellent cosmetic results in selected small, superficial lesions. Depending on the location and size of the primary lesion, radiation treatment can be accomplished by external beam radiation therapy (EBRT) or brachytherapy, or a combination of both. Cartilage invasion per se is not a contraindication for RT, because the risk of necrosis is low with fractionated irradiation.51
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree