CANCERS THAT AFFECT MEN AND WOMEN
Bladder
The most common presentation of bladder cancer is in the form of transitional cell carcinoma, and although most cases tend to be superficial when first diagnosed, repeated treatments may be required to treat recurrences. Treatments for bladder cancer mainly include surgery, radiation therapy, immunotherapy, and chemotherapy, with surgery used in most of the cases. From a sexual health perspective, treatment of bladder cancer by radical cystectomy is the procedure that has received the most attention given its likely negative implications on sexual functioning.133 The efficacy of laparoscopic surgery in terms of oncologic control has been recently studied,134–136 but it is not clear if it differentially impacts sexual functioning.137
Male Sexual Function
A standard radical cystectomy for treating bladder cancer in men involves the removal of the bladder as well as the prostate, seminal vesicles, vasa deferentia, and the removal or damage of the neurovascular bundles, leaving the patient with the likely consequent loss of sexual function.138 Alternatively, a nerve-sparing cystectomy is associated with a temporary decrease in function after surgery, commonly followed by a return to function.139–143 Unfortunately, oncologic failure rates for nerve-sparing cystectomies are higher.144–146 This has resulted in a debate regarding the minimization of cancer risk, while maximizing sexual function preservation.142,147–150 In addition, men report body image issues as a result of their need for urinary diversion following surgery,133,151 which is associated with sexual dissatisfaction.152
For men who are treated with radical or nerve-sparing cystectomy, erectile dysfunction prevails at similar rates to those seen after prostatectomy or nerve-sparing prostatectomy, respectively, and its treatment follows the standard of care for erectile dysfunction in men treated with a prostatectomy. Early PDE-5 inhibitor intervention is associated with improved sexual function and satisfaction.153 However, almost half of male bladder cancer patients do not seek treatment for their sexual dysfunction postsurgery.138,154
Female Sexual Function
The standard radical cystectomy for bladder cancer in women involves the removal of the bladder, urethra, anterior vaginal wall, uterus, and ovaries and it is likely to result in sexual dysfunction,155–157 including weakened libido, dyspareunia, decreased lubrication, and diminished ability or inability to achieve orgasm.158,159 Consequently, many women who undergo non–nerve-sparing cystectomies report discontinuing sexual intercourse following surgery.157 The use of nerve-sparing surgical procedures has recently been evaluated in an attempt to reduce the impact of treatment on the significant sexual dysfunction caused by a radical cystectomy.157 Women who undergo nerve-sparing surgery retain sexual function comparable to presurgical levels,141,157,160 as well as increased potential of fertility preservation.161 However, there has been limited exploration of the oncologic impact of nerve-sparing surgeries for these women.
Treatment for sexual dysfunction amongst female bladder cancer survivors is similar to recommendations for female survivors of breast, gynecologic, and colorectal cancers. For women who experience physical and/or psychological difficulties resulting from the extent of their cystectomy, vaginal reconstruction surgery may be a viable option.162
Head and Neck
Head and neck cancers are considered to be among the most impactful with regard to the patient’s posttreatment quality of life. Treatment for head and neck cancer can include surgery, radiation therapy, targeted therapy, and/or chemotherapy. Even when function-sparing approaches are utilized, treatment can still result in facial alterations, as well as changes to saliva quality and/or quantity, breathing, and speech,163 which cannot be completely corrected with follow-up treatments.164,165 Head and neck cancer survivors report lowered self-esteem and body image as a result of these treatment-related side effects, which negatively impact their intimate relationships.166–168 These survivors report feeling less attractive, a reduced libido, and decreased overall satisfaction with their sexual relationships.169–173 In particular, females, and those who were diagnosed with more advanced stage disease, who underwent surgery, or whose treatment caused more extensive disfigurement are at elevated risk for sexual dysfunction.169,174,175
The majority of these patients do not discuss implications of their disease and treatment on body image and sexual health with their medical providers.172 Sexual health rehabilitation is not well understood in this population.176,177 Open communication about possible sexual side effects of treatment between providers and patients is associated with improved psychosocial adjustment.175,178
Blood, Bone Marrow, and Lymphatic System
Treatment of systemic disease often involves intense interventions with chemotherapy, whole-body irradiation, and/or bone marrow transplant and may cause side effects, including sexual dysfunction. In particular, preparative regimes of high-dose chemotherapy and total body irradiation may result in ovarian failure and low-estrogen levels for women, and gonadal and cavernosal arterial insufficiency in men.179 Sexual problems in bone-marrow transplant patients may still be present several years after the procedure and include difficulty obtaining an erection, ejaculation, and orgasm for men and concerns about body appearance, vaginal dryness, and painful intercourse and orgasm for women.180 Moreover, a longitudinal study of sexual function in long-term survivors of hematopoietic cell transplantation documented that at 5 years after treatment, sexual dysfunction is a major problem for this population, with male survivors continuing to have lower sexual function and female survivors having lower scores for sexual activity and function compared to controls.181 Another important issue that often develops for females after transplant is genital graft-versus-host disease (GVHD). Female genital GVHD after transplant is a complication that tends to receive little attention despite the significant impact it has on the survivor’s quality of life. Female tract GVHD may manifest in several forms, from irritation of the vagina or the vulva to ulceration and vaginal stenosis, and it has been found to be present in at least 25% of the transplant survivor population.182,183 Research studies suggest that an early detection of genital GVHD is highly treatable with steroids and vaginal dilators, and therefore, education on active self-surveillance should be given to patients in addition to a plan for regular gynecologic follow-up posttransplant.183,184
Studies that investigate the effectiveness of interventions for sexual dysfunction in women after bone-marrow transplantation are scarce, but therapies including vaginal lubricants, dilators, and vibrators can be recommended to improve some of the sexual side effects of treatment. In men, there is evidence to support that a number of patients recover sexual function over time,181 and that testosterone cypionate and sildenafil have shown to improve erectile dysfunction in this population.179 More importantly, many survivors and their partners may feel hesitant or even fearful to resume sexual activity after bone marrow transplantation given concerns about immunosuppression, such as coming in contact with germs. These concerns may prevail even after survivors have been cleared by their medical team to resume sexual activity. It is important, therefore, to anticipate and openly discuss these concerns with survivors and their partners while offering gentle guidance and reassurance about their eligibility to resume sexual activity.
Colorectal
Treatments for colorectal cancer mainly include surgery, radiation therapy, and chemotherapy, with surgery used in most of the cases.9 Colorectal cancer surgery often causes damage to the sympathetic and parasympathetic nerves and results in erectile and ejaculatory disorders in men and dyspareunia, decreased libido, and changes in the orgasm experience in women.185 And although the impact of radiotherapy in colorectal cancer survivors has rarely been addressed, the available research suggests that radiation is associated with sexual dysfunction in both men and women.186
Because most colorectal cancer patients are over 50 years of age and may presumably have a higher incidence of non–cancer-related sexual dysfunction, studies that use a longitudinal design to assess the prevalence of sexual dysfunction in these survivors are of particular interest. In a longitudinal study by Jayne et al.,187 the authors compared treatment outcomes in patients who underwent a laparoscopic-assisted surgery versus open surgery for colorectal cancer, including quality of life and sexual functioning. The authors assessed functioning preoperatively and at 2 weeks and 3, 6, 18, and 36 months after surgery and found that although there was no change from baseline in sexual functioning or enjoyment for men and women in both arms, men tended to report more sexual problems from 3 months onward in the laparoscopic arm and from 6 months onward in the open surgery arm. The authors also found that body image was worse than at baseline from 2 weeks onward for all patients. Therapies recommended for prostate cancer survivors, such as the use of sildenafil,188 appear to be adequate for a number of male colorectal cancer survivors who suffer from erectile dysfunction following treatment. Likewise, therapies recommended for breast and gynecologic cancer survivors, such as the use of water-based lubricants, vaginal moisturizers, and vaginal dilators, can also be recommended for female colorectal cancer survivors who suffer from vaginal dryness and/or stenosis after radiation.186 More specific to colorectal cancer survivors are the negative emotional reactions to the colostomy, such as poor body image and reduced self-esteem, which are commonly present and may negatively impact intimacy. But patients and their partners can and do learn how to manage the impact of an ostomy on sexuality, and there are resources available for this purpose. Patients with ostomies should receive information on deodorants to minimize odor, as well as on foods that are likely to cause stronger odors, gas, or diarrhea. Patients should also receive information on pouch covers, and suggestions such as changing positions to avoid pain during intercourse and emptying the stoma before sexual activity.189 Additional information for patients and their partners can also be found through the United Ostomy Associations of America, Inc.
Breast
Difficulty with sexual function, loss of desire, changes in body image, and disruption of emotional relationships are primary sexual complications of breast cancer from diagnosis through all stages of treatment and into survivorship. Chemotherapy, radiation, surgery, and adjunctive hormonal therapy, whether delivered alone or in combination with each other, all have the potential to negatively impact sexual function. The survival rates of breast cancer for localized disease continue to improve and are now 95% or better.9 Not only will the majority of breast cancer survivors go on to become long-term cancer survivors, but studies have also shown that the majority of partnered women will remain sexually active either after diagnosis or at the end of treatment.190,191
A number of factors have been identified regarding who is at risk for developing sexual problems after breast cancer. Women who have premorbid sexual dysfunction,192 negative self-concept,193 depression, and relationship discord194 are all more likely to struggle with sexual problems. In one recent prospective study assessing the impact of breast cancer on women’s sexuality, a younger age was found to be the most salient predictor of lower sexual function in addition to a lack of partner status.195 Unfortunately, there is a wide range of sexual problems that are related to breast cancer, and reports of sexual problems range from 30% to 100%.196 More specifically, 23% to 64% of women report problems with desire, 20% to 48% report arousal or lubrication problems, 16% to 36% report problems with orgasm, and 35% to 38% report problems with pain or dyspareunia.197–199 Problems with body image are also quite common both during and after treatment, although there is some evidence that certain areas of distress such as feeling feminine or perceived attractiveness tend to improve over time.190,200,201
Surgery
Conceptually, losing one or both breasts would seem to be one of most dramatic ways to damage a woman’s core sense of femininity, body integrity, and attractiveness. Concern about body image in the face of breast surgery and potential breast changes and breast loss is prevalent with approximately 30% to 67% of women reporting concern with body image.194,202 Breast-conserving surgical procedures and reconstructive surgery have become a standard part of breast cancer care, and it is generally assumed that breast-conserving surgery (lumpectomy versus mastectomy) as well as breast reconstruction is essential in helping women maintain a positive body image.203 Several studies have shown that women who undergo modified radical mastectomies have poorer body image than those who have had breast-conserving procedures. In Moyer’s meta-analysis204 of 40 studies comparing quality of life differences between breast-conserving surgery and mastectomy between 1980 and 1995, it was shown that patients undergoing breast conservation had a better body image compared to mastectomy patients.
However, aside from body image and maintaining body integrity, breast surgery can still have a significantly negative impact on sexuality. In particular, women who undergo breast reconstruction are typically left with a complete lack of sensation, including nipple sensation. The nipple has been shown to be the most sensitive area of the breast and loss of nipple sensation is akin to losing a key erogenous zone for many women.205 Although their breast shape may be restored, the loss of feeling is not. In addition to the use of saline-filled or silicone gel–filled implants, women may also have tissue flap procedures where tissue from a woman’s body may be harvested from her abdomen, back, thighs, or buttocks in order to reconstruct a breast. These surgeries, although more intensive than implant surgery, offer the advantage of reconstruction that often feels and looks more natural without the concern about implant rupture or the need to replace implants over time. However, flap procedures necessitate a second surgical site and additional scars. There is growing attention now being paid to the use of nipple-sparing mastectomy; however, although the nipple and areola may be left in place and breast tissue is removed, sensation is no longer intact.206
Chemotherapy
Disruption of sexual function after breast cancer seems to be significantly related to whether a woman undergoes chemotherapy as part of her treatment.190,194,196,197,200,207 Ganz191 looked at the impact of breast cancer treatment on sexual function and found that women who had either a lumpectomy or mastectomy followed by chemotherapy were more likely to report negative sexual outcomes than patients who had surgery alone. Schover207 has noted that younger women who undergo abrupt chemotherapy-related menopause are at the highest risk for sexual problems, and that the rates of sexual dysfunction in these women are clearly higher than would be expected in a healthy, community-based sample. In particular, the intensive estrogen deficiency that comes with chemotherapy-induced menopause often leads to severe vaginal dryness and vaginal atrophy, which makes penetration painful. Painful intercourse due to vaginal dryness is one of the most common sexual problems after breast cancer, and it is one of the primary factors also implicated in women’s experience of decreased desire, another common and often vexing problem for breast cancer survivors.207,208 Testosterone deficiency related to premature ovarian failure has also been discussed as a factor related to a loss of desire after breast cancer, but research has not uniformly supported this hypothesis.209,210 Rather, a loss of desire appears to be multidimensional, with causes that span a bio–psycho–social continuum.
Hormonal Therapy
Endocrine therapy including selective estrogen receptor modifiers (SERM) and aromatase inhibitors (AI) now play an important role in breast cancer treatment for both premenopausal as well as postmenopausal women. Tamoxifen has been used as systemic adjuvant treatment for over 20 years and primary side effects are hot flashes, fatigue, and nausea. Regarding sexual function, tamoxifen use has been associated with vaginal dryness and low desire,211 although in the large-scale Breast Cancer Prevention Trial, there were no differences found in the frequency of sexual activity between those using tamoxifen versus placebo.212 Raloxifene, another SERM, does not appear to confer a significant difference from tamoxifen in patient-reported outcomes on physical health and depression, but sexual function has been reported as being slightly better than in women taking tamoxifen.213 More recently, sexual side effects of raloxifene treatment have been examined and no deleterious effects were found.214 The third-generation AIs (anastrozole, letrozole, and exemestane) have become an integral component in the care of postmenopausal women with estrogen-receptor–positive breast cancer and are currently being evaluated for use in chemoprevention.215 A significant and distressing exacerbation of postmenopausal gynecologic symptoms such as extreme vaginal dryness and dyspareunia has been reported in several studies investigating the quality of life of women taking AIs, including in the first and largest Arimidex and Tamoxifen Alone or in Combination (ATAC) trial with over 9,000 participants.216 It has been noted that as use of AIs has become the gold standard of care for postmenopausal breast cancer survivors, it is imperative to explore comprehensive management of these gynecologic symptoms that impair sexual function.217
Radiotherapy
Radiation therapy for breast cancer is generally localized to the breast. Radiation can result in skin fibrosis, additional loss of sensitivity in the skin, and fatigue, all which can contribute to low desire. However, there has hardly been any research conducted that specifically examines the effects of breast cancer–related radiotherapy on sexuality.
Intervention
Several approaches for addressing sexual problems after breast cancer have been identified. Most approaches focus on individually based information and education about the management of sexual side effects, such as vaginal dryness. The minority of interventions have been developed aimed at working with couples to establish new norms for intimacy after cancer. Ganz and colleagues218 demonstrated that nurse-delivered individually based counseling was more successful in managing menopausal and sexual side effects from treatment over a 4-month period than usual care. Alternatively, it has been suggested that interventions need to actively involve women’s partners in order to produce lasting benefits on sexual functioning.219 It is our perspective that optimal intervention is essentially based on “two tracks” and addresses sexuality in both an individual and relational context.
Track 1: Focus on the Individual. It is imperative that women receive information and education about how to maintain and restore good sexual health in the context of maintaining good vaginal health as well as overall well-being after breast cancer. In anticipation of the common side effects of both chemotherapy-induced menopause as well as adjuvant hormonal therapies, we believe that all women should receive information as part of their overall treatment planning about (1) nonhormonal vaginal moisturizers, (2) water-based vaginal lubricants, (3) pelvic floor strengthening (Kegel) exercises, and (4) the value of maintaining blood flow to vaginal tissue to prevent vaginal atrophy. This kind of information is readily available to patients through a number of resources, including a free, recently updated booklet by the American Cancer Society called “Sexuality and Cancer: For the Woman Who Has Cancer and Her Partner.” This booklet is free to patients and can be obtained in hard copy or online. In addition, the National Cancer Institute as well as the Lance Armstrong Foundation have information about sexuality on their Web sites.
Track 2: Focus on the Relational Context. For the majority of breast cancer patients who are in a partnered relationship, it may also be important to acknowledge that sexuality is experienced in a context. Often, patients do not realize that partners may benefit from looking at some of the same educational resources, and many popular books and Web sites about sexuality after breast cancer actually have sections that are specifically written for partners. It can be a great relief to patients to get the message that not only are sexual problems common after breast cancer, but also that although the majority of partners want to be helpful when it comes to reconnecting sexually, they too may be unsure of how to proceed and may need guidance. For patients that are not currently in a relationship, sexuality is, in part, still a relational experience whether based on past relationships or in the context of hopes for future relationships. Patients who are not partnered are frequently unsure of how to proceed in terms of dating and lack of confidence in initiating new sexual relationships after their treatment. Often, these patients gain enormous benefit from being able to talk about these challenges and strategize about communication with a new potential partner.
Gynecologic Cancers
Treatments for gynecologic cancer often result in sexual dysfunction that may affect a substantial number of patients and can persist for many years after diagnosis. The effect of surgeries on the genitals can impact a patient’s self-esteem and body image and can create significant physical barriers, such as pain, to satisfactory sexual experiences. In addition, women who receive radiation therapy are at risk for developing vaginal fibrosis and stenosis, and hormonal interventions are likely to result in an abrupt development of menopausal symptoms, all of which can considerably disrupt sexual functioning.220 For instance, Carmack et al.221 found that sexual problems were quite prevalent in ovarian cancer patients, with 80% reporting problems with vaginal dryness, 75% reporting problems reaching orgasm, and 62% reporting pain or discomfort during penetration. Moreover, research has found that ovarian cancer survivors report significantly less sexual pleasure than disease-free controls.222
In a cross-sectional study of women diagnosed with early-stage cervical carcinoma, Bergmark et al.223 noted that long-term survivors reported sexual function changes that appear to persist over time, including decreased lubrication and genital swelling during arousal, reduced perceived elasticity during intercourse, and distress over these changes. Moreover, the authors223 found that the changes in sexual function reported by the survivors were associated with the effects of surgery, whether or not the treatment included radiotherapy. Later results obtained by Jensen et al.,224 who studied early-stage cervical cancer survivors longitudinally, suggest that although low sexual interest and vaginal dryness seem to persist for years after a radical hysterectomy, other changes such as distress by a reduced vaginal size and problems completing sexual intercourse after surgery are likely to resolve over time. In contrast, research also suggests that women treated with radiation for advanced, recurrent, or persistent cervical cancer report sexual problems including low sexual interest, lack of lubrication, dyspareunia, and inability to complete sexual intercourse throughout the first 2 years after treatment and with little improvement over time.225
Even though most women who are diagnosed with cervical cancer are younger, Bergmark et al.223 found that women of all ages included in their study are likely to consider sexuality an important aspect of their lives, and therefore, providers should feel particularly encouraged to discuss with their patients possible disease- and treatment-related sexual changes that may occur.
There is limited information of the sexual outcomes associated with vulvar cancer treatment. However, the available research suggests that vulvar cancer survivors experience significant sexual dysfunction.226,227
Intervention
Research studies on interventions that may be used for women treated for gynecologic cancers and who experience subsequent sexual dysfunction are limited, and mostly focus on information giving and suggestions to manage specific symptoms.228 For instance, for women who experience vaginal dryness, silicone-based or water-based lubricants may be recommended. The use of vaginal dilators a few times per week, and even in combination with Kegel exercises, help stretch the vaginal tissue after radiation. As a general recommendation, vaginal dilation should start as soon as the woman is comfortable, but usually within 4 weeks after completion of radiotherapy.228 Of note, research has suggested that psychoeducational interventions that combine information with motivational and behavioral skills are more effective than information alone in improving adherence to the use of vaginal dilators in younger women treated for gynecologic cancer.229 Moreover, psychoeducation may be particularly useful at decreasing some of the distress associated with sexual changes following gynecologic cancer treatment.230 For women who experience vaginismus after radiation therapy, a combined approach of pharmacotherapy and sex therapy should be recommended.228 In addition, information should be given to patients on techniques to restore the blood flow to the clitoris and the vagina.231 It is important to also consider that sexuality is more than the ability to complete sexual intercourse. For survivors who feel unable to have intercourse, either due to physical or psychological factors, counseling may facilitate the exploration and broadening of sexual and intimate interactions that feel comfortable to the survivors and their partners.
BRCA Mutation Carriers
Related to the sexual problems that both breast and gynecologic cancer patients face, women with a BRCA1 or BRCA2 mutation represent a vulnerable population that has received very little attention regarding sexual dysfunction. The hereditary cancer genes, BRCA1 and BRCA2, confer a remarkably high lifetime risk of both breast (55% to 85%) and/or ovarian cancer (15% to 44%).232 A prophylactic bilateral mastectomy (PBM) has been shown to reduce the risk of breast cancer in mutation carriers by over 90%,233 and a prophylactic bilateral salpingo-oophorectomy (BSO) significantly reduces both ovarian cancer risk over 80% and the risk of breast cancer by at least 50%.234 Although BSO and PBM are the most effective options to reduce cancer risk in BRCA carriers, their impact on sexual functioning should not be underestimated. It is recommended that female BRCA1 carriers have their ovaries and fallopian tubes removed prophylactically by age 35 to 40 years, whereas BRCA2 carriers may be able to defer this surgery until their mid 40s because the average time for developing ovarian cancer in BRCA2 carriers is somewhat later. However, for both groups, recommendations for BSO is long before the average age of natural menopause, meaning that these women will face an abrupt, surgically induced onset of menopause with all of the related sexual side effects such as vaginal dryness and irritation, pain with penetration, decreased arousal, and loss of desire. Like breast cancer patients who undergo breast surgery, mutation carriers who opt for PBM will face similar issues regarding surgical scars, loss of sensation, and changes in perceived self-image secondary to potentially significant body changes. However, unlike other cancer patients, often, BRCA carriers identify their genetic mutation in a context in which they themselves do not have cancer, and may have to wrestle with the decisions about surveillance versus risk-reducing surgery with moderate to little support and a paucity of adequate counseling and guidance, including regarding management of sexual side effects of surgery.235
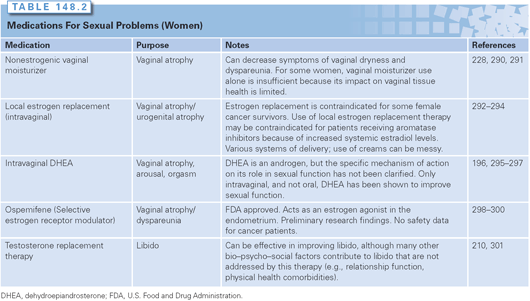
Regarding intervention, BRCA carriers should receive the same information and education regarding the management of premature menopause as is given to other cancer patients, including recommendations for managing vaginal dryness using vaginal moisturizers and water-based lubricants. In contrast to women who have already had breast cancer, it has been shown that short-term hormone replacement therapy does not appear to negate the risk-reduction benefit gained by prophylactic surgery,236 thus making systemic hormone replacement as well as localized vaginal estrogen both reasonable options to be explored (Table 148.2).
CANCER IN CHILDREN AND YOUNG ADULTS
Cancer during childhood and adolescence can have a tremendous impact on the patient’s psychosexual development.237 Treatment puts these young patients at risk for significant sexual dysfunction and infertility,238–242 as well as impairing their normal social development, which can result in increased isolation, more limited sexual behavior (e.g., less frequent masturbation, communication with friends about sex), longer delays before dating, and decreased interest in and satisfaction with sex.243–246 In light of these potential challenges in their psychosexual maturation, about one-third of survivors report at least one distressing long-term sexual problem, with females more likely to report sexual dysfunction.247 Problems with sexual function are associated with poorer psychological and quality of life.247,248
Adolescent and young adult cancer survivors do not receive adequate age-appropriate information and counseling regarding issues related to their sexual development.246,249–251 Limited intervention research has demonstrated that a psychosocial intervention can help to improve sexual knowledge and body image and can decrease anxiety related to sexual issues.252 Furthermore, survivors report limited (and, often, inaccurate) knowledge about fertility issues following treatment,253,254 which is concerning because the majority of patients who are childless at diagnosis report a desire for future offspring.255 For both genders, there are medical options for fertility preservation that should be discussed with the patient as soon as is feasible.256
RELEVANT SOCIOCULTURAL CONSIDERATIONS
It is now well documented that specific racial and ethnic groups in the United States have a higher risk for developing certain cancer diagnoses that directly affect sexual organs. For instance, African American men have a higher risk of developing prostate cancer compared to Caucasians,257 and Latinas have twice the risk of developing cervical cancer compared to non-Hispanic Caucasians.258 But beyond gaining knowledge on the cancer facts and figures for different racial and ethnic groups, becoming aware of the impact that culture has on the development of sexual beliefs, attitudes, and practices may be one of the best tools for providers when understanding and helping cancer patients of diverse backgrounds who are coping with sexual dysfunction.
Guidelines for what constitute normative sexual behaviors and gender-specific role prescriptions are greatly influenced by consensus within particular communities,259 and it has been found that certain prescribed gender roles can intensify sexual problems.260 The following are some of the differences that have been observed in sexual attitudes and quality of life in cancer patients and survivors of diverse ethnicities and their implications for medical practice.
Special Considerations for Male Patients
Recent findings on issues related to sexual functioning for African American prostate cancer patients report results that are worth noting. Although having erectile dysfunction is likely to lead to psychological consequences for men across races and cultures, it has been found that African American men are significantly more likely than Caucasian men to consider sexual side effects when choosing treatment for prostate cancer.261 In the same study by Jenkins et al.,261 African American men were more likely than their Caucasian counterparts to indicate that an erection is an essential element to sex and to seek help for sexual problems. According to Johnson et al.,262 African American men, in spite of showing better recovery of sexual function at 12 and 60 months after diagnosis and treatment with prostatectomy, were more likely than non-Hispanic Caucasians to report that sexual function continued to be a moderate to big problem. Knowledge of these reported differences, and specific factors such as the fact that African American prostate cancer patients seem likely to have positive attitudes about seeking treatment for an erectile problem, may assist providers in framing a culturally sensitive discussion with prostate cancer patients of African American origin with regard to their options for treatment and management of sexual side effects.
Special Considerations for Female Patients
A few studies document special considerations for minority, female cancer patients, and survivors with regard to their sexual functioning. For African American breast cancer survivors, particular concerns about body image are reported, such as keloid formation and total body hair loss,263 and body image concerns in general appear to be greater for African American than for Caucasian women.264 Although findings suggest that African American breast cancer survivors view their sex lives as less disrupted by cancer compared to their Caucasian counterparts,265 feeling sexually attractive has been found to be predictive of subsequent psychological well-being for this population.266 Similar to breast cancer survivors of other ethnicities, African American breast cancer survivors report a need to receive more information from their health-care providers regarding sexual dysfunction as a possible side effect of treatment.263 However, research suggests that African American women may prefer to address their sexual health concerns in a one-to-one context rather than in group settings.263,266 Moreover, a randomized trial of peer counseling in African American breast cancer survivors documented that brief psychoeducational interventions can be effective at addressing informational needs on sexual health for this population.267
For Latino, Asian, and Native American women, the literature on sexual outcomes in cancer treatment and appropriate interventions for treatment-related sexual dysfunction continues to be limited. However, Asian and Latina cervical cancer survivors have reported great concern with the effects of treatment on their appearance, with Latinas expressing more negative feelings about the impact of treatment on their bodies and their relationships compared to Asian, African American, and Caucasian survivors.268 Moreover, cultural factors such as the language barrier for Latinas,268 and the perception of sex as taboo for women from many Asian societies269 ought to be considered as prevalent impediments in accessing services and to address treatment-related sexual dysfunction. In spite of these challenges, however, the available research findings suggest that psychoeducational interventions may be appropriate for Latinas,270 and factual information on sexuality after cancer, preferably delivered by nurses or doctors, is often sufficient and acceptable to Asian and African American women cancer survivors as a way to address their concerns.263,271
DISRUPTION OF INTIMACY AND RELATIONAL CONSIDERATIONS
In addition to the myriad of physiologic changes that can disrupt sexual function after cancer diagnosis, it is important to acknowledge the significant interpersonal shifts that may take place and subsequently affect sexuality. For instance, women’s perceptions of their partner’s reactions to their appearance after cancer consistently predict their own acceptance of their self-image and of their sense of femininity.272
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








