Sentinel Lymph Node Biopsy
Alice P. Chung
Armando E. Giuliano
INTRODUCTION
Axillary lymph node dissection (ALND) has been an integral component of the staging, prognosis, and treatment of invasive breast cancer and is discussed in Chapter 38. Surgical management of the axilla, however, has undergone a paradigm change since the concept of lymphatic mapping of the breast was introduced at the John Wayne Cancer Institute (JWCI) in 1991, and sentinel lymph node biopsy (SNB) has replaced ALND for axillary staging in clinically node-negative early breast cancer. Although tumor characteristics and molecular markers are increasingly contributing to the understanding of the biology of breast cancer, the status of the axilla remains the most important prognostic indicator for overall survival. The current algorithm for axillary management of invasive breast cancer incorporates outcomes information from SNB, ALND, and axillary irradiation as well as data from the effects of systemic therapies on axillary metastases. This chapter addresses the current understanding of the role of SNB in surgical management of the axilla for breast cancer.
SENTINEL NODE CONCEPT IN CANCER
In the early 1970s, Kett et al. (1) reported that the first regional lymph node, the “Sorgius node,” could be identified in breast cancer using direct mammalymphography. This was a cumbersome technique that required a formal ALND to isolate the suspected lymph nodes, radiographic evaluation of the resected nodes to identify the suspicious ones, and determination of concordance through histopathologic confirmation.
Ramon Cabanas (2) coined the term sentinel node as a specific lymph node group in penile carcinoma, located in a constant anatomic location in the pelvis. The sentinel node (SN) concept evolved from this observation of specific anatomic nodal drainage and postulates that a primary tumor is drained by an afferent lymphatic channel that courses to the first, “sentinel,” lymph node in that specific regional lymphatic basin (3). If the tumor has metastasized, it will do so to this node. The tumor status of the SN reflects the tumor status of the nodal basin. Morton et al. (4) tested the hypothesis that the SN in a given regional basin can be identified
by an indicator dye in a feline model and then validated it in the clinical setting in a group of patients with melanoma.
by an indicator dye in a feline model and then validated it in the clinical setting in a group of patients with melanoma.
Identification of a Sentinel Node in Breast Cancer
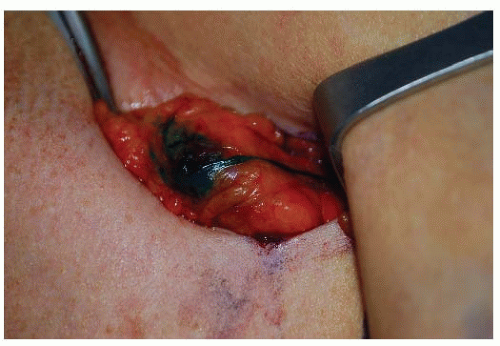
The feasibility of identifying an SN intraoperatively in breast cancer was first investigated at the JWCI by Giuliano et al. (5). In October 1991, the authors’ group began to investigate the feasibility of lymphatic mapping and sentinel lymphad-enectomy with isosulfan blue vital dye in breast cancer as a more accurate and less morbid approach to stage breast cancer (Fig. 37-1). This prospective study demonstrated that SNB of the axilla is technically feasible, safe, and without added complications. With a defined technique and experience, a 100% accuracy to predict the status of the axilla was subsequently achieved (5, 6). In addition to vital dye-directed lymphatic mapping, three other technical approaches for SN identification in breast cancer with accuracy rates comparable to the blue dye have evolved: radio-guided surgery, radio-guided surgery with preoperative lymphoscintigraphy, and the combination of vital dye and isotope techniques. The most commonly used agents are isosulfan blue dye and filtered technetium sulfur colloid. An increased SN identification rate with the use of the combination of blue dye and radioisotope is well documented. However, there has been only one prospective randomized trial comparing blue dye alone to the combined use of isotope and blue dye, and in this study Morrow et al. showed no difference in SN identification between the two groups (7). The authors found the number of cases performed by an individual surgeon to be the most significant predictor of successful SN identification, demonstrating that surgeon experience impacts SN identification and false-negative rates. Experienced surgeons are extremely successful in accurately identifying the SN regardless of technique. SN identification in breast cancer is technically feasible, safe, and an accurate predictor of the status of the axilla using several different technical approaches.
Proof of Principle
The SN hypothesis for breast cancer has been tested in the clinical setting by several groups of investigators who performed complete histopathologic evaluation of the SN and non-SNs using the same pathologic processing with step sectioning, hematoxylin and eosin (H&E) and immunohistochemistry (IHC) for all H&E negative axillary lymph nodes (8). Turner and colleagues identified 33/103 (32%) patients with a tumor-bearing SN by H&E. IHC evaluation of 157 negative SNs upstaged 10 patients (14.3%). In 60 patients whose SNs were negative by H&E and IHC, 1,087 non-SNs were examined at two levels by IHC and only one additional tumor-positive node was identified. In 57.3% of patients the SN was negative. In the 44 patients with a tumor-positive SN, 56.8% had involvement of the SN alone. Additional studies, including an NCI-sponsored multicenter trial that examined all non-SNs with the same rigorous histopathologic analysis, reported similar findings for cases with negative SN that had further evaluation of non-SN with IHC (9). The SN concept has been validated by these studies enabling widespread clinical application of this technique.
LYMPHATIC ANATOMY OF THE BREAST AND IMPLICATIONS FOR SENTINEL NODE IDENTIFICATION
Anatomy
The axilla is bordered by the latissimus dorsi posteriorly, the axillary vein superiorly, the chest wall medially, the pectoralis muscles anteriorly and extends laterally to where the vein crosses between the lateral edge of the pectoralis major and latissimus dorsi muscles. Level I nodes are located inferior and lateral to the pectoralis minor muscle, level II nodes posterior to the pectoralis minor and below the axillary vein, and level III nodes are medial to the pectoralis minor and below the clavicle. Lymphatic drainage generally follows an orderly sequential pattern from level I to level II nodes and rarely to level III. SNB is a staging procedure that removes one or more lymph nodes from the axillary basin. The SN is found in level I in 83% of cases, level II in 15.6%, in level III in 0.5%, internal mammary in 0.5%, supraclavicular in 0.1%, and elsewhere in 0.3% (10).
Patterns of Regional Nodal Drainage
The axilla is the primary site of drainage in about 95% of breast cancer cases, with isolated internal mammary drainage seen in less than 5% (10). Primary drainage to other nodal pathways, such as supraclavicular, cervical, intercostal, and contralateral lymph nodes, is extremely uncommon. Lymphoscintigrams can accurately identify nodal uptake of radioisotope preoperatively (Fig. 37-2).
Although the axilla is the primary drainage site, with other regions receiving limited lymphatic flow, the prognostic value of the internal mammary nodal status is high, particularly when both axillary and internal mammary nodes are either negative with better survival than with either basin having metastases or with the worst prognosis when both basins are involved (11). In those rare cases, with small tumors and sole drainage to nodal stations other than the axilla, identification of tumor positive regional nodes may be important for adjuvant therapy recommendations or to determine external beam irradiation fields. There have been several groups who have investigated the impact of internal mammary nodal drainage identified by preoperative lymphoscintigraphy on outcome. Kong et al. (12) reviewed their database of 1,172 patients with stage I to III invasive breast cancer who had
preoperative lymphoscintigrams following peritumoral injection of radiocolloid and identified 334 patients with drainage of radiocolloid to the internal mammary nodes. These patients were significantly younger, less likely to have upper outer quadrant tumors, and more likely to have smaller and medial tumors than patients without drainage to the internal mammary nodes. Rates of internal mammary irradiation did not differ between the two groups. With median follow-up time of 7.4 years, internal mammary drainage was significantly associated with a worse distant disease-free survival (DFS)but not locoregional recurrence or overall survival. The data from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial demonstrated that the majority of positive SNs came from levels I and II of the ALND, and only 1.2% of the positive SN specimens came from non-axillary locations (13). In general, nodal status is becoming less relevant in determining adjuvant therapy.
preoperative lymphoscintigrams following peritumoral injection of radiocolloid and identified 334 patients with drainage of radiocolloid to the internal mammary nodes. These patients were significantly younger, less likely to have upper outer quadrant tumors, and more likely to have smaller and medial tumors than patients without drainage to the internal mammary nodes. Rates of internal mammary irradiation did not differ between the two groups. With median follow-up time of 7.4 years, internal mammary drainage was significantly associated with a worse distant disease-free survival (DFS)but not locoregional recurrence or overall survival. The data from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial demonstrated that the majority of positive SNs came from levels I and II of the ALND, and only 1.2% of the positive SN specimens came from non-axillary locations (13). In general, nodal status is becoming less relevant in determining adjuvant therapy.
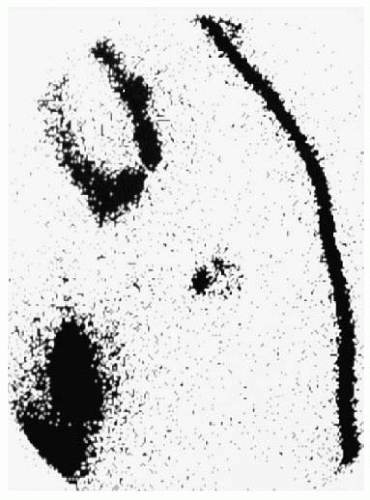 FIGURE 37-2 Preoperative lymphoscintigraphy demonstrates a left anterior oblique view of a sentinel node. |
Early anatomists studied the lymphatics of the breast by injecting mercury into the lactiferous ducts of female cadavers (14). In 1874, Sappey concluded that all of the lymphatics arising from the breast drained to the axilla via the subareolar plexus. As techniques for visualizing the lymphatics have evolved, our understanding of the lymphatic drainage in the breast has changed (14). Analysis of the superficial and deep lymphatic anatomy of the breast and upper torso from human cadaver studies supports the SN concept. Suami and colleagues (15) studied 24 breasts in 14 human cadavers and found that superficial lymphatic collectors drain into the same first-echelon node close to the lateral edge of the pectoralis minor muscle (Fig. 37-3). In most cases, the drainage was to only one SN. In several cases, however, there was at least one other first-echelon node from a collecting lymphatic that passed directly through the breast. The investigators also found that lymphatics of the nipple-areola complex are different from other areas of the breast and drain only into the first-echelon pectoral node shown in Figure 38-3 in green, but not to the depicted orange node that receives lymphatic drainage that passes through the breast. This study did not identify any direct anastomosis between the superficial collecting system and the collectors associated with internal mammary vessels, an observation consistent with the findings of studies that have used lymphoscintigraphy to study patterns of lymphatic drainage by site of injection (16).
The findings in Suami’s anatomic study may explain the clinical experience with lymphatic mapping and the persistence of a false-negative (FN) rate of 8% to 11% (13, 16). Dye injected deep into the parenchyma along the purple-colored track (depicted in Fig. 37-3) reaches both the depicted green and orange lymph nodes in the pectoral group, whereas dye injected into the subareolar or intradermal location reached only the depicted green node. SNB using the intraparenchymal injection technique would track to both nodes and suggests that the intraparenchymal route of injection may be more likely to track to both first-echelon nodes and result in a more accurate staging of the axilla. The proof of principle study discussed above was performed with the intraparenchymal injection method and had one FN node (8).
Pan et al. (17) used combined preservation techniques with computed tomographic lymphangiography to obtain a three-dimensional analysis of the lymphatics in the bilateral breasts and anterior upper torso of a human cadaver. They found a predominance of superficial lymphatics with radial drainage to the axilla, and asymmetry between the right and left breasts with high variation in number and size of lymphatic vessels between sides.
Blumgart and colleagues (18) evaluated lymphoscintigraphic data from 2,304 breast cancer patients (2,284 female, 20 male). All patients received four peritumoral injections of technitium 99m sulfur colloid followed by lymphoscintigraphy with documentation of drainage patterns. Unlike Pan, they found that lymphatic drainage and tumor distribution were symmetric between both breasts. There were no differences in drainage patterns between males and females. They also found that among 2,304 patients, axillary (2,263, 98.2%), interpectoral (n = 15, 0.7%), internal mammary (n = 813, 35.5%), infraclavicular (n = 25, 1.1%) and supraclavicular (n = 70, 3.0%) nodal fields directly drain the breasts with variable frequencies, and that patients usually drained to one nodal field (64%) but it was possible for drainage to occur to multiple nodal fields (36%).
Cumulative Experience of Sentinel Node Identification for Staging
Investigators from academic centers and the surgical community worldwide have introduced SNB into clinical practice as a staging procedure. Several multicenter lymphatic mapping trials have confirmed the feasibility of SNB as a staging procedure and reported data on identification and FN rate (Table 37-1) (13, 19, 20). The identification rate ranges from 86% to 97% with accuracy from 96% to 98% and a FN rate from 4% to 16.7%. Most of the multicenter trials required some formal instruction or validation prior to participation of each institution, however early on most surgeons were self-taught. SN identification rate and accuracy are highly dependent on surgeon experience, more so than technique.
The NSABP B-32 trial randomized patients with clinically node-negative invasive breast cancer to either SNB followed by a level I or II ALND (group 1), or observation of the axilla if the SN was tumor free (group 2) (13). Five thousand six hundred eleven women were randomly assigned to the treatment groups, 3,986 had pathologically negative SN with follow-up information. The study reported an SN identification rate of 97.2%, accuracy rate of 97.1%, and a FN rate of 9.8%. With a mean follow-up of 95.6 months, there was no significant difference between the two groups with respect to overall survival, disease-free survival, and regional control (21).
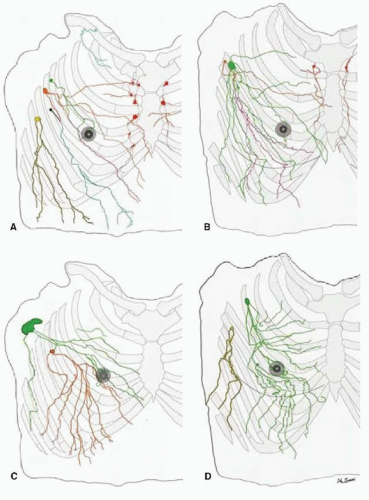 FIGURE 37-3 Tracing distally of lymphatics of both hemi upper torsos (male: (A) and (C), female: (B) and (D)) from each first-tier lymph node color coded: pectoral node (green, orange, black, and yellow), subclavicular node (light blue), and internal mammary node (red). Note: that the lymph collecting vessels from the nipple and areolar region on each specimen drain into the green-colored lymph node; the similar pattern of chest and breast drainage between the male and female studies; the breast lies in the pathway of collecting lymphatics that start peripherally; and although the majority of the breast drains to one sentinel node in D, every breast area is drained by more than one first-tier node in each study. (From Suami H, Pan W-R, Mann GB, et al. The lymphatic anatomy of the breast and its implications for sentinal lymph node biopsy: a human cadaver study. Ann Surg Oncol 2008;(3):863-871.) |
TABLE 37-1 Identification Rate and False-Negative Rate of Selected Multicenter Sentinel Lymph Node Trials That Evaluated the Status of the Axilla with Sentinel Lymph Node Dissection Followed by Completion Axillary Lymph Node Dissection | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
The Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial was a two-phase trial that required surgeons to demonstrate a 90% identification rate and a FN rate of less than 5% prior to proceeding to phase II, which was the two-armed prospective trial that randomized 1,031 patients into SNB followed by ALND (n = 516) or to SNB alone (n = 515) if the SN was tumor free (20). If the SN was positive for tumor cells, the regional treatment was ALND or axillary irradiation. SNB was performed with a 96% success rate with the combined use of blue dye and radioisotope and a 5% FN rate. The investigators reported a significantly lower rate of lymphedema, sensory deficits, and impairment in shoulder function in the SNB arm with patient-recorded quality of life scores statistically significantly better in the SNB arm. A report on long-term outcomes is pending.
The Royal Australian College of Surgeons (RACS) SN versus Axillary Clearance (SNAC) multicenter randomized study was a phase III trial with a two stage design similar to the ALMANAC trial. A sensitivity of 95%, FN rate of 5%, and a negative predictive value of 98% were reported for SN biopsy in stage I. Stage II randomized 1,088 clinically node-negative women with invasive breast cancer less than 3 cm to SNB alone versus axillary clearance to compare rates of axillary morbidity. The average increase in arm volume was 2.8% in the SNB alone group and 4.2% in the axillary clearance group (p = .002). Patients in the SNB alone group gave lower ratings for arm swelling (p < .001), symptoms (p < .001), and dysfunctions (p = .02), but not disabilities (p = .5) (19).
HISTOPATHOLOGIC PROCESSING
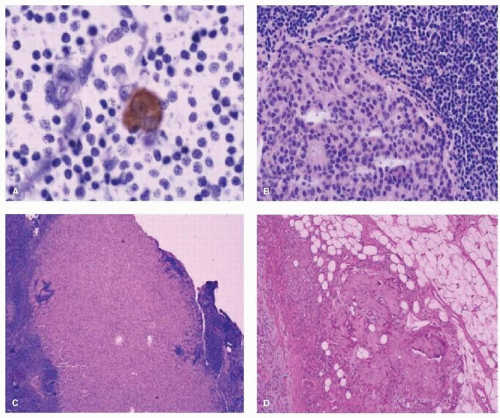
When the authors’ group at the JWCI compared ALND alone to SNB followed by completion ALND, axillary metastases were identified in 29% of the ALND-alone group compared to 42% in the SNB group (p < .03) (22). H&E analysis of multiple levels of the SN increased the sensitivity to detect micrometastases for SNB versus ALND (9.2% vs. 3.0%, respectively; p < .004), and when both H&E and IHC were used, there was increased sensitivity (16.0% vs. 3.0%, respectively; p < .0005) (Fig. 37-4). Focused histopathologic analysis of the SN is a more sensitive method to detect micrometastases by both H&E and IHC and leads to improved accuracy of axillary staging for a tumorpositive axillary lymph node. The hope was that ultra-staging of the SN would lead to the identification of H&E node-negative patients who were at higher risk for recurrence.
In view of the identification of small tumors in the SN, the American College of Pathologists established guidelines to process the SN with frozen sections, imprint cytology, or permanent formalin processed specimens (23). The SN is bivalved along the longitudinal axis, serially sectioned at 1.5 to 2.0 mm thickness blocks and each block is sectioned at three levels. If metastases are identified in the SN (see Fig. 37-4), the size of the metastasis is reported as macrometastases (>2.0 mm), micrometastases (>0.2 and ≤2.0 mm), or isolated tumor cells (≤0.2 mm), and the method of detection of the metastasis by H&E, IHC, or reverse transcription-polymerase chain reaction (RT-PCR). In the new American Joint Committee on Cancer (AJCC) guidelines (seventh edition), small clusters of cells not greater than 0.2 mm, or nonconfluent or nearly confluent clusters of cells not exceeding 200 cells in a single histologic cross section of a lymph node are classified as isolated tumor cells.
Molecular analysis of the SN is an area of emerging technology and interest. Some investigators feel that this is a more objective assessment of the tumor burden in the SN, is more reproducible, can be standardized, and evaluates more tissue in a shorter period of time (24). Quantitative RT-PCR (qRT-PCR) evaluation demonstrates a 98% accuracy and can be performed in 40 minutes or less. A prospective trial to evaluate lymph node metastases with a multiplex RT-PCR-based assay detected 98% of metastases greater than 2 mm and 88% of those greater than 0.2 mm, and results were superior to frozen section histology or imprint cytology (24). In a prospective multicenter trial that conducted molecular analysis of SN by qRT-PCR as well as serial sectioning and staining with H&E and with or without IHC in 547 patients, investigators compared the two groups with respect to clinical outcome with mean follow-up of 7 years (25). While molecular staging of SN detected more nodal metastases not seen by standard histologic evaluation, these metastases were not shown to be a significant predictor of disease recurrence. Similar results were observed by Fisher and colleagues (26) who analyzed seven breast cancer associated genes, known to be overexpressed in metastatic breast cancer, in axillary lymph nodes of 501 patients with T1-T3 invasive breast cancer who were followed for 5 years with no impact on clinical outcome. Molecular analysis of SN is unlikely to be of clinical relevance in view of recently reported results of the ACOSOG Z10 and NSABP-B32 trials on micrometastases, discussed later in this chapter. In the absence of participation in a clinical trial, the reasonable management approach should be based on the H&E evaluation of axillary nodes; however, in clinical practice IHC is used in many centers for SNs that are found negative by H&E.
FACTORS INFLUENCING THE SUCCESS AND ACCURACY OF SENTINEL LYMPH NODE DISSECTION
In order to reduce the FN rate of SNB, causes of failure have been sought. Potential explanations for failure include improper surgical technique, lack of surgeon and pathologist experience, lymphatic physiology, aberrant lymphatic patterns, and patient and tumor characteristics.
Effect of Sentinel Lymph Node Dissection Technique on Accuracy
A variety of technical factors, which include type of dye or radioisotope, filtered versus unfiltered isotope, timing of surgery after injection, and site of injection (peritumoral, subdermal, intradermal, subareolar) influence the performance of SNB. A high degree of accuracy and a low FN rate with resection of only one or two lymph nodes is seen in most cases. Differences in the ability to find the SN are reflections of variations in patient characteristics (e.g., obesity, age) and surgeon experience more than technique. Internal mammary nodes are visualized less often with intradermal injection than with peritumoral injection. Subareolar injection of isotope offers some advantages over peritumoral injection; for example, when the tumor is nonpalpable, it increases the distance from the injection site of radioisotope to the axilla for upper outer quadrant lesions, reducing the shine through, and is a good choice for multicentric disease.
In the multi-institutional American College of Surgeons Oncology Group (ACOSOG) Z0010 trial, 198 surgeons enrolled 5,237 patients and used blue dye with radiocolloid in 79.4% of cases, blue dye alone in 14.8%, and radiocolloid alone in 5.7% with a success rate of 98.7%, corresponding to a failure rate of 1.7% (27). The percent of failed SNB with blue dye was 1.4%, radiocolloid 2.3%, and the combination 1.2% (p = .2813). The number of cases (≤50 compared to >50) enrolled was associated with a statistically significant failure rate. Increased body mass index and age were also associated with decreased SN identification in this study. Morrow et al. evaluated isosul-fan blue dye alone and compared it to dye with isotope (7). Surgeons achieved equal results with either method. A study from New Zealand confirms this work and reports that identification of the SN is similar with blue dye alone compared to a triple modality approach (lymphoscintigraphy, intraoperative gamma probe, and intraoperative blue dye) (28). The blue dye had an accuracy of 98% and a sensitivity of 96% compared to the triple method accuracy of 95% and sensitivity of 91%. There has been a manufacturing shortage of isosulfan blue in the United States and methylene blue has been used as an alternative vital dye. The success rate of methylene blue with radioisotope is reported to be equivalent to isosulfan blue with isotope (29).
The site of injection of the tracer may influence the outcome of SNB. A prospective randomized trial compared intradermal, intraparenchymal, and subareolar routes of injection and demonstrated a significantly higher rate of localization and more rapid transit by lymphoscintigraphy, and shorter time to surgery with the intradermal injection (16). Another randomized multicenter trial compared periareolar and peritumoral injection of radiotracer and blue dye (30). The intraoperative success was similar for blue dye or gamma detection (99.1%). The detection rate was higher for the periareolar site for each tracer, but this was not statistically significant. The SN was blue in 94.7%, hot in 97.1%, and both in 92.6%. The concordance was 91.5% with the peritumoral injection and 95.6% with the periareolar injection. The blue dye and radiocolloid concordance for the positive SN patients was 94.5% and, when assessed by site of injection, 96.2% in the periareolar group and 92.9% in the peritumoral group.
The SNB procedure has been adopted by surgeons in both academic and community settings throughout the United States as well as internationally. The efficacy of the method, dye, isotope, or both, is more likely a reflection of training and experience than variations in the success of the method itself. The importance of quality control and appropriate training cannot be overemphasized.
Effect of Surgeon Experience: Training and Performance of Sentinel Lymph Node Dissection
Formal lymphatic mapping instruction with hands-on experience leads to a 90% to 95% identification rate and a 3.8% to
4.3% FN rate when more than 30 cases are performed (31). The NSABP B-32 trial required a minimum of five prequalifying cases and reported a technical success rate of 97% (13). Surgical volume impacts identification rates. Surgeons who performed fewer than three cases per month had a success rate of 86.23% ± 8.30%, for three to six cases 88.73% ± 6.36%, and for six or more SN biopsies 97.81% ± 0.44% (31).
4.3% FN rate when more than 30 cases are performed (31). The NSABP B-32 trial required a minimum of five prequalifying cases and reported a technical success rate of 97% (13). Surgical volume impacts identification rates. Surgeons who performed fewer than three cases per month had a success rate of 86.23% ± 8.30%, for three to six cases 88.73% ± 6.36%, and for six or more SN biopsies 97.81% ± 0.44% (31).
The studies just described show individual variation in learning the skills and identify some of the pitfalls in learning the technique. Instruction in SNB is now part of surgical residency training in the United States. For those not trained in the technique during residency, formal instruction, use of dual agents, performance of approximately 20 SNB procedures with a backup ALND, and an adequate volume of cases to maintain skills are all factors that contribute to successful identification of SN and reduced FN rate. In 2005, a consensus statement from the American Society of Breast Surgeons suggests that prior to abandoning ALND for a negative SN, 20 cases of SNB be performed with an identification rate of 85% and a FN rate of 5% or less (32). These should be adapted on an individual basis, with more cases performed by those with lower identification rates and higher FN rates and vice versa. One problem with their application is that most patients are SN negative, making the FN rate more difficult to determine with a high degree of certainty.
Effect of the Number of Sentinel Nodes Removed
Increasing the mean number of SNs removed may improve accuracy (13, 33). The number of SNs removed statistically affected the FN rate in the NSABP B-32 trial, where a median of 2 SNs was removed in each treatment arm. The FN rate was 17.7% when one node was removed, 10% for two, 6.9% for three, 5.5% for four, and 1% for five or more nodes removed. In the University of Louisville Breast Cancer Sentinel Lymph Node Study, the mean number of SNs removed per patient was 2.2 and 58% of the patients had multiple SNs removed (33). The overall SN identification rate was 90% with an 8.3% FN rate. If a single node was removed, the FN rate was 14.3% compared to 4.3% when multiple SNs were removed (p < .0004). The first two or three SNs removed predict the status of the axilla in about 98% of cases, but additional positive SNs will be identified when four or more nodes are removed, improving the FN rate (13). The removal of all blue, or radioactive, nodes with a count equal to or greater than 10% of the most radioactive node has been shown to decrease the FN rate in these studies. This increase in staging accuracy may be obtained at the cost of an increased rate of complications, especially lymphedema, but the goal is accurate staging for treatment decisions. Fewer nodes may be removed with increasing surgeon experience.
Effect of Patient and Tumor Characteristics
The data from the multi-institutional, randomized prospective NSABP B-32 trial reports a FN rate of 9.8% and an overall accuracy of 97.1% (13). Differences in tumor location (inner and central location vs. lateral and outer), no hot spot identified preoperatively, small tumor size, older age, and type of diagnostic biopsy (excision/incisional biopsy higher than fine-needle aspiration [FNA] or core needle biopsy [CNB]) increased the FN rate. In the ALMANAC study, increased body mass index (BMI), upper outer quadrant location, and nonvisualization on lymphoscintigraphy were significantly associated with failed identification (p < .001, p = .008, p < .001, respectively) (34). None of the following, age, tumor size, tumor histology, tumor grade, or multifocality, affected identification. In the ACOSOG Z0010 trial, a higher failure to identify a SN occurred with increased BMI and age of 70 or older (27).
Impact of Sentinel Lymph Node Dissection on Regional Control and Survival for Sentinel Node-Negative Patients
Results from randomized controlled trials examining local recurrence after SNB alone are summarized in Table 37-2. A meta-analysis of 48 studies that included 14,959 SN-negative patients followed for a median of 34 months demonstrated an axillary failure in 67 patients (0.3%) (35). In the European Institute of Oncology Trial the predicted failure was eight cases, but only one case of overt axillary metastases was seen at 7.2 years of follow-up after surgery. The possibility that the occult metastases in the FN nodes may never become overt has been suggested by several single institution studies, the Swedish multicenter trial, and from the European Institute of Oncology trial at a relatively short follow-up.
One multicenter trial to report results on patients randomized to SNB alone or SNB followed by ALND was the Sentinella/GIVOM trial (36). This study reported a FN rate of 16.7%. Despite this high FN rate, there was only one axillary failure in the SNB-alone group at 55.6 months. The overall survival was 95.5% in the ALND group and 94.8% in the SNB-alone group at 5 years of follow-up.
In the European Institute of Oncology randomized study, women with tumors less than 2 cm were randomized to SNB
alone if the SN was tumor free or to SNB followed by ALND (37). In the ALND group, 32% had a positive SN and 8 of 174 SN-negative patients had a FN node. The SN was positive in 36% of the SNB-only group with one axillary failure at a median follow-up of 79 months. Because there were eight FNs in the ALND group, there should have been eight FNs in the SNB alone group, but there was only one axillary failure. The overall survival was the same for the ALND group compared to SNB alone (96.4% vs. 98.4%, respectively; p = .6). The SNB-alone group had decreased morbidity and cost.
alone if the SN was tumor free or to SNB followed by ALND (37). In the ALND group, 32% had a positive SN and 8 of 174 SN-negative patients had a FN node. The SN was positive in 36% of the SNB-only group with one axillary failure at a median follow-up of 79 months. Because there were eight FNs in the ALND group, there should have been eight FNs in the SNB alone group, but there was only one axillary failure. The overall survival was the same for the ALND group compared to SNB alone (96.4% vs. 98.4%, respectively; p = .6). The SNB-alone group had decreased morbidity and cost.
TABLE 37-2 Comparison of Outcomes in Five Randomized Controlled Trials of Sentinel Lymph Node Biopsy versus Axillary Lymph Node Dissection
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|