This article summarizes the relative pros and cons surrounding the timing of sentinel lymph node (SLN) biopsy in patients undergoing neoadjuvant chemotherapy. Several institutions initiated prospective trials of SLN biopsy performed after neoadjuvant chemotherapy in conjunction with a completion axillary lymph node dissection, with the goal of ultimately showing that SLN biopsy could be safely and accurately performed after the patient completed systemic therapy. Other institutions adopted a policy of performing SLN biopsy before initiation of chemotherapy. This avoided the issue surrounding the accuracy of SLN biopsy after chemotherapy and potentially provided information that might influence adjuvant therapy decisions. This article addresses the clinical questions regarding the 2 approaches including the accuracy of the procedure and the prognostic information gleaned.
There are few innovations in surgery that have so rapidly and drastically changed the management of a disease as the introduction of sentinel lymph node (SLN) biopsy has changed breast surgery. Beyond the obvious of greatly minimizing the morbidity associated with the surgical staging of breast cancer, our knowledge of the anatomy of the breast and axilla and the biology of breast cancer metastasis has improved. However, no great advance comes without great controversy, and it is almost stunning how a procedure so beautifully simple in concept has led to such vigorous debate. Among several areas of debate, perhaps the most controversial is that of the timing of SLN biopsy in the patient receiving neoadjuvant therapy.
Before the introduction of sentinel lymph node biopsy as a method of staging the axilla, there was little consequence surgically on whether patients received neoadjuvant chemotherapy or not, as either way they would be receiving an axillary lymph node dissection. The most significant impact of preoperative therapy on axillary staging was that there were going to be patients who may have been node positive initially but were node negative after chemotherapy and so their true pretreatment nodal status was never known. At the time, however, this would not alter their surgery and the associated risks, nor did it significantly impact therapy decisions.
This changed dramatically as lymphatic mapping and SLN biopsy assumed greater prominence in the surgical therapy of breast cancer. Now patients who opted for neoadjuvant chemotherapy to shrink their primary tumor and potentially avoid mastectomy were obligated to undergo axillary lymph node dissection (ALND) as part of their surgery, whereas if they had a mastectomy, they would be candidates for SLN biopsy and potentially avoid ALND. This put patients in the awkward position of having to decide which was the lesser of 2 evils: losing the breast or the increased risk of lymphedema.
In addition to the surgical dilemma, the controversy was further fueled by the increasing desire, and ability, to tailor care to the individual patient. The nodal status of the patient took on increased importance in deciding whether adjuvant chemotherapy was indicated, what regimen, and whether radiation therapy would be recommended after mastectomy. As the question of how to best incorporate SLN biopsy into the neoadjuvant paradigm became more critical, 2 approaches emerged. Several institutions initiated prospective trials of SLN biopsy performed after neoadjuvant chemotherapy in conjunction with a completion ALND, with the goal of ultimately showing that SLN biopsy could be safely and accurately performed after the patient completed systemic therapy. Other institutions adopted a policy of performing SLN biopsy before initiation of chemotherapy. This avoided the issue surrounding the accuracy of SLN biopsy after chemotherapy and potentially provided information that might influence adjuvant therapy decisions.
This article summarizes the relative pros and cons surrounding the timing of SLN biopsy in patients undergoing neoadjuvant chemotherapy ( Table 1 ), as well as addresses the clinical questions regarding the 2 approaches including the accuracy of the procedure and the prognostic information gleaned.
| SLN Biopsy Before Chemotherapy | SLN Biopsy After Chemotherapy | |
|---|---|---|
| Accuracy |
|
|
| Convenience |
|
|
| Prognosis |
|
|
| Outcome |
|
|
Potential advantages to SLN biopsy before neoadjuvant chemotherapy
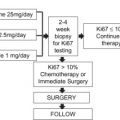
The first approach is to perform the SLN biopsy before beginning chemotherapy. Although this requires an additional surgery, and could potentially delay the initiation of chemotherapy, the strongest argument for this approach is the perceived accuracy of the procedure. When SLN biopsy was first introduced there was some concern as to the accuracy of the procedure in the presence of a large primary tumor, which constituted most patients being considered for neoadjuvant therapy. However, 2 series looking specifically at SLN biopsy in this patient population demonstrated that this was not a concern. Several institutions that adopted pretreatment SLN biopsy subsequently reported their results with this specific approach ( Table 2 ). Given the documented safety of avoiding ALND for a negative SLN biopsy, patients who are found to be SLN negative before treatment can safely avoid ALND at the time of their surgical therapy. This is in contrast with concerns raised regarding the accuracy of SLN biopsy after chemotherapy, as is discussed in more detail later in this article. With very little data on regional recurrence rates among patients who have a negative SLN biopsy after neoadjuvant chemotherapy, without completion ALND, the risks of this approach remain unclear. Performing SLN biopsy before chemotherapy avoids these concerns.
| First Author | Year | N | ID Rate | SLN Positive | Patients With Positive NSLN After Chemotherapy |
|---|---|---|---|---|---|
| Zirngibl | 2002 | 15 | 93% | 43% | 0% |
| Schrenk | 2003 | 21 | 100% | 43% | 66% |
| Sabel | 2003 | 26 | 100% | 52% | 70% |
| Ollila | 2003 | 22 | 100% | 45% | 40% |
| Jones | 2005 | 52 | 100% | 58% | NR |
| Van Rijk | 2006 | 25 | 100% | 44% | 40% |
| Cox | 2006 | 47 | 98% | 83% | 65% |
| Grube | 2008 | 55 | 100% | 55% | 45% |
| Papa | 2008 | 86 | 99% | 67% | NR |
| Straver | 2009 | 75 | 100% | 29% | 32% |
The other primary advantage of performing SLN biopsy is that the true pretreatment nodal status of the patient is known. This is important if the nodal status might affect the decision to give chemotherapy at all, the regimen used, or the decision to give chest wall radiation if the patient ultimately requires mastectomy or include the regional nodes in the radiation fields. Whether this is a true advantage or not is controversial, as this represents a moving target. The optimal agents and regimens used for systemic therapy are constantly evolving. For example, several years ago when this controversy was first emerging, the addition of a taxane to an anthracycline-based regimen, or the use of trastuzumab (Herceptin) in the face of Her-2/neu overexpression, was limited to node-positive patients as there were few data in the node-negative population. Thus there was a greater importance of the pretreatment nodal status in deciding what regimen to use. As more data emerged regarding the benefits in the node-negative population, the pretreatment nodal status became less important. For most patients being considered for neoadjuvant therapy, the clinical tumor size and information from the core biopsy became sufficient to select therapy, and the importance of the SLN status before chemotherapy diminished.
The pendulum began to swing back when Oncotype DX (Genomic Health, Redwood City, CA, USA) was introduced. Now genomic profiling could be used to identify hormone-receptor–positive, node-negative patients who, despite a large-sized tumor, received very little benefit from adjuvant chemotherapy, and may ultimately be better served with neoadjuvant hormonal therapy. Therefore, in some patients, knowing the nodal status might be crucial to neoadjuvant therapy decisions. The ground may shift again as new data emerge suggesting that Oncotype DX may still be useful in identifying patients who can avoid chemotherapy even if they are node positive, or as new agents are introduced into the adjuvant/neoadjuvant setting. The increasing use of immunohistochemical staining, genomics, and possibly proteomics in the selection of targeted therapies may continue to reduce the importance of SLN status.
Potential advantages to SLN biopsy after neoadjuvant chemotherapy
If patients have an SLN biopsy before neoadjuvant chemotherapy, they are committing themselves to at least 2 operations: 1 before chemotherapy and at least 1 after chemotherapy. This deviates from the current convention of neoadjuvant treatment where all of the chemotherapy is completed, followed by all of the surgery. If SLN biopsy can be safely performed after neoadjuvant chemotherapy, a greater percentage of patients can complete their surgical therapy in 1 trip to the operating room. And, whereas breast cancer patients are regularly asked to commit to more than 1 operation (patients undergoing SLN biopsy know they may need to return to the operating room [OR] for an ALND, patients undergoing breast-conserving therapy [BCT] know they may need to return to the OR for a reexcision lumpectomy or mastectomy), in today’s health care economic climate, the need to conserve costs is becoming increasingly important. In addition, depending on the surgeon’s schedule, the time required to schedule and perform the SLN biopsy may delay the onset of chemotherapy.
A more significant criticism of performing SLN biopsy before neoadjuvant therapy is that it commits more patients to an ALND even though the chemotherapy may have eradicated any remaining disease from the axilla. Performing the SLN biopsy after chemotherapy takes advantage of the potential downstaging effect of chemotherapy on axillary nodes. In the reported series of SLN biopsy before chemotherapy, the SLN was positive in between 30% and 80% of patients, all of whom then had completion ALND in addition to either lumpectomy or mastectomy at the completion of systemic therapy ( Table 3 ). Some patients who would have a positive SLN biopsy before neoadjuvant therapy and thus require ALND may have a negative SLN biopsy following therapy and not require a complete node dissection, further minimizing the morbidity of treatment. Studies of patients with cytologically confirmed axillary metastases before neoadjuvant chemotherapy have reported pathologic complete response (pCR) in the axillary nodes in 20% to 36% of patients, and it is feasible that patients with micrometastases in the SLN may have a higher rate of pCR.
| First Author | Year | N | ID Rate | SLN Positive | FN Rate |
|---|---|---|---|---|---|
| Cohen | 2000 | 38 | 82% | 52% | 17% |
| Breslin | 2000 | 51 | 84% | 51% | 12% |
| Nason | 2000 | 15 | 87% | 69% | 33% |
| Fernandez | 2001 | 40 | 90% | 59% | 20% |
| Tafra | 2001 | 29 | 93% | 52% | 0% |
| Stearns | 2002 | 34 | 85% | 45% | 14% |
| Brady | 2002 | 14 | 93% | 77% | 0% |
| Julian | 2002 | 34 | 91% | 39% | 0% |
| Miller | 2002 | 35 | 86% | 30% | 0% |
| Vigario | 2003 | 37 | 97% | 50% | 28% |
| Balch | 2003 | 32 | 97% | 58% | 5% |
| Reitsamer | 2003 | 30 | 87% | 54% | 7% |
| Schwartz | 2003 | 21 | 100% | 48% | 9% |
| Piato | 2003 | 42 | 98% | 56% | 17% |
| Haid | 2003 | 45 | 93% | 45% | 5% |
| Kang | 2004 | 54 | 72% | 62% | 11% |
| Shimazu | 2004 | 47 | 94% | 66% | 12% |
| Lang | 2004 | 53 | 94% | 47% | 4% |
| Patel | 2004 | 42 | 95% | 42% | 0% |
| Aihara | 2004 | 20 | 85% | 35% | 17% |
| Kinoshita | 2005 | 77 | 93.5% | 33% | 18% |
| Mamounas | 2005 | 428 | 85% | 36% | 11% |
| Jones | 2005 | 36 | 80.6% | 55% | 11% |
| Tanaka | 2006 | 70 | 90% | 90% | 5% |
| Lee | 2007 | 219 | 78% | 69% | 5.6% |
| Papa | 2008 | 31 | 87% | 59% | 16% |
| Tausch | 2008 | 167 | 85% | 42% | 8% |
| Gimbergues | 2008 | 129 | 93.8% | 46% | 14.3% |
| Hino | 2008 | 55 | 71% | 46% | 0% |
| Classe | 2008 | 195 | 90% | 23.5% | 11.5% |
| Hunt | 2009 | 84 | NR | NR | 5.9% |
| Shwartz | 2010 | 79 | 98.7% | 29% | 4.1% |
A third potential advantage to SLN biopsy after chemotherapy is that the information may be more useful to the practitioner than the pretreatment nodal status. Proponents of pretreatment SLN biopsy argue that knowing the SLN status may help guide treatment and having a negative SLN biopsy after chemotherapy does not allow you to know whether the patient was node positive to begin with. However, knowing the response of the disease in the axilla may be more important in determining risk of recurrence, and the decision to proceed with postmastectomy radiation, than knowing the nodal status before treatment. If a patient has a positive SLN biopsy before chemotherapy, and a negative ALND afterward, it is often unclear whether there was additional disease that responded or whether all nodal disease was removed with the first operation. Proponents of SLN biopsy after chemotherapy would argue that the presence or absence of disease in the nodes after chemotherapy may be a better criterion by which to choose which patients should receive postmastectomy radiation than the nodal status before chemotherapy.
Potential advantages to SLN biopsy after neoadjuvant chemotherapy
If patients have an SLN biopsy before neoadjuvant chemotherapy, they are committing themselves to at least 2 operations: 1 before chemotherapy and at least 1 after chemotherapy. This deviates from the current convention of neoadjuvant treatment where all of the chemotherapy is completed, followed by all of the surgery. If SLN biopsy can be safely performed after neoadjuvant chemotherapy, a greater percentage of patients can complete their surgical therapy in 1 trip to the operating room. And, whereas breast cancer patients are regularly asked to commit to more than 1 operation (patients undergoing SLN biopsy know they may need to return to the operating room [OR] for an ALND, patients undergoing breast-conserving therapy [BCT] know they may need to return to the OR for a reexcision lumpectomy or mastectomy), in today’s health care economic climate, the need to conserve costs is becoming increasingly important. In addition, depending on the surgeon’s schedule, the time required to schedule and perform the SLN biopsy may delay the onset of chemotherapy.
A more significant criticism of performing SLN biopsy before neoadjuvant therapy is that it commits more patients to an ALND even though the chemotherapy may have eradicated any remaining disease from the axilla. Performing the SLN biopsy after chemotherapy takes advantage of the potential downstaging effect of chemotherapy on axillary nodes. In the reported series of SLN biopsy before chemotherapy, the SLN was positive in between 30% and 80% of patients, all of whom then had completion ALND in addition to either lumpectomy or mastectomy at the completion of systemic therapy ( Table 3 ). Some patients who would have a positive SLN biopsy before neoadjuvant therapy and thus require ALND may have a negative SLN biopsy following therapy and not require a complete node dissection, further minimizing the morbidity of treatment. Studies of patients with cytologically confirmed axillary metastases before neoadjuvant chemotherapy have reported pathologic complete response (pCR) in the axillary nodes in 20% to 36% of patients, and it is feasible that patients with micrometastases in the SLN may have a higher rate of pCR.
| First Author | Year | N | ID Rate | SLN Positive | FN Rate |
|---|---|---|---|---|---|
| Cohen | 2000 | 38 | 82% | 52% | 17% |
| Breslin | 2000 | 51 | 84% | 51% | 12% |
| Nason | 2000 | 15 | 87% | 69% | 33% |
| Fernandez | 2001 | 40 | 90% | 59% | 20% |
| Tafra | 2001 | 29 | 93% | 52% | 0% |
| Stearns | 2002 | 34 | 85% | 45% | 14% |
| Brady | 2002 | 14 | 93% | 77% | 0% |
| Julian | 2002 | 34 | 91% | 39% | 0% |
| Miller | 2002 | 35 | 86% | 30% | 0% |
| Vigario | 2003 | 37 | 97% | 50% | 28% |
| Balch | 2003 | 32 | 97% | 58% | 5% |
| Reitsamer | 2003 | 30 | 87% | 54% | 7% |
| Schwartz | 2003 | 21 | 100% | 48% | 9% |
| Piato | 2003 | 42 | 98% | 56% | 17% |
| Haid | 2003 | 45 | 93% | 45% | 5% |
| Kang | 2004 | 54 | 72% | 62% | 11% |
| Shimazu | 2004 | 47 | 94% | 66% | 12% |
| Lang | 2004 | 53 | 94% | 47% | 4% |
| Patel | 2004 | 42 | 95% | 42% | 0% |
| Aihara | 2004 | 20 | 85% | 35% | 17% |
| Kinoshita | 2005 | 77 | 93.5% | 33% | 18% |
| Mamounas | 2005 | 428 | 85% | 36% | 11% |
| Jones | 2005 | 36 | 80.6% | 55% | 11% |
| Tanaka | 2006 | 70 | 90% | 90% | 5% |
| Lee | 2007 | 219 | 78% | 69% | 5.6% |
| Papa | 2008 | 31 | 87% | 59% | 16% |
| Tausch | 2008 | 167 | 85% | 42% | 8% |
| Gimbergues | 2008 | 129 | 93.8% | 46% | 14.3% |
| Hino | 2008 | 55 | 71% | 46% | 0% |
| Classe | 2008 | 195 | 90% | 23.5% | 11.5% |
| Hunt | 2009 | 84 | NR | NR | 5.9% |
| Shwartz | 2010 | 79 | 98.7% | 29% | 4.1% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree