Neoadjuvant endocrine therapy improves surgical outcomes for postmenopausal women with bulky hormone receptor–positive breast cancer. Recent studies indicate that this approach may also be used in the management of smaller tumors with the hope of predicting outcomes from adjuvant endocrine manipulation. Neoadjuvant endocrine therapy provides a unique opportunity to identify molecular predictors of endocrine responsiveness and agents that can be used in combination with endocrine therapy to improve tumor response and overcome endocrine resistance.
The emergence of neoadjuvant systemic therapy has allowed surgically inoperable breast cancers to become operable and has increased the rate of breast-conserving surgery in those who would otherwise require a mastectomy. In premenopausal women, neoadjuvant chemotherapy is the current standard. However, in postmenopausal women with estrogen receptor (ER)-positive breast cancer, preoperative endocrine therapy with aromatase inhibitors (AIs) improves rates of breast conservation and minimizes treatment-related toxicities. In addition, the ability to obtain tumor specimens before and after neoadjuvant endocrine therapy has facilitated the development of biomarkers and gene profiles predictive of endocrine responsiveness and the evaluation of novel agents to improve the outcome of ER-positive breast cancer.
Tamoxifen versus surgery
Early studies in the 1980s and 1990s focused on endocrine therapy use with tamoxifen as an alternative to surgery in patients aged 70 years and older who were poor surgical candidates. Although tumor shrinkage with tamoxifen was demonstrated, long-term disease control was poor. A trial by the European Organization for Research and Treatment of Cancer (EORTC) randomized postmenopausal women aged 70 years and older to modified radical mastectomy versus tamoxifen 20 mg daily. They found that there was a higher rate of local progression or relapse in the tamoxifen-treated arm, but no difference in survival. This and other studies paved the way for a shift in treatment strategy to preoperative endocrine therapy before surgery, rather than in place of it.
AIs versus tamoxifen
AIs have been found to be superior to tamoxifen as endocrine therapy for postmenopausal women with hormone receptor–positive breast cancer in the settings of metastatic disease, locally advanced disease, and early-stage disease. The molecular mechanism explaining the benefit of AIs compared with tamoxifen is still not clear, but may be related to the difference in the mechanism of action between the 2 classes of drugs or pharmacogenomic variables that differentially affect drug metabolism, or both. For example, AIs block the conversion of androgens to estrogens in the peripheral tissues, thereby depriving the ER of its agonist. On the other hand, tamoxifen directly binds to the ER and acts as an agonist or antagonist in various tissues. Although the CYP2D6 genotype may be implicated in the efficacy of tamoxifen in some studies, the debate continues and germ line polymorphisms that affect AI efficacy are still being explored. Regardless, the improved outcomes with AIs compared with tamoxifen in postmenopausal women with ER+ breast cancer prompted several trials to be designed to compare these agents in the neoadjuvant setting.
One of the first trials to compare an AI with tamoxifen in the neoadjuvant setting was the P024 trial. This was a randomized, double-blind, multicenter study that randomized 324 postmenopausal women with ER+ and/or progesterone receptor (PgR)-positive breast cancer to letrozole 2.5 mg daily or tamoxifen 20 mg daily for 4 months. At enrollment none of the patients were felt to be candidates for breast-conserving surgery (BCS) and 14% of the patients were inoperable. In the intention-to-treat analysis there was a statistically significant improvement in the overall objective response rate (by clinical palpation) in the letrozole group compared with the tamoxifen group (55% vs 36%; P <.001). All secondary end points were also found to be significant between the 2 groups: ultrasound response, 35% versus 25% ( P = .042), mammographic response, 34% versus 16% ( P <.001), and BCS, 45% versus 35% ( P = .022). Few pathologic complete responses (pCR) were seen in the primary breast lesions in either group with only 2 in the letrozole group and 3 in the tamoxifen group.
The Pre-Operative Arimidex Compared to Tamoxifen (PROACT) trial compared anastrozole with tamoxifen in postmenopausal women with ER+ and/or PgR+ operable or potentially operable invasive breast cancer. This was a randomized, double-blind, double-dummy, multicenter study that randomized 451 patients to 3 months of anastrozole 1 mg daily or tamoxifen 20 mg daily. No significant difference in response rates was observed in the 137 patients who received concomitant neoadjuvant chemotherapy. After excluding those patients who received chemotherapy, similar response rates were seen in the anastrozole (36.2%) and tamoxifen (26.5%) groups ( P = .07). The objective response rates for anastrozole and tamoxifen were 48.6% and 35.8% (caliper-measured; P = .04), respectively, and 36.6% and 24.2% (ultrasound-measured; P = .03) in patients who were not eligible for BCS or were inoperable at baseline. Improvement in actual surgery occurred in 43% of anastrozole-treated patients and 31% of tamoxifen-treated patients ( P = .04).
Another neoadjuvant endocrine therapy trial, the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) trial, was a phase III randomized, double-blind, double-dummy, multicenter trial that randomized patients to anastrozole 1 mg and tamoxifen placebo daily, tamoxifen 20 mg and anastrozole placebo daily, or a combination of tamoxifen 20 mg and anastrozole 1 mg daily for 12 weeks before surgery. There were 330 postmenopausal women with invasive ER+, nonmetastatic, and operable or locally advanced potentially operable breast cancer included in the study. Objective response rates were 37%, 36%, and 39%, respectively, based on caliper measurement and 24%, 20%, and 28%, respectively, on ultrasound. These differences were not statistically significant. Of the patients felt to be candidates for BCS by their surgeons, a statistically significant number received anastrozole compared with tamoxifen (46% vs 22%, P = .03). There was a trend toward improved numbers of BCS in patients in the anastrozole arm compared with the tamoxifen arm, although this was not significant (44% vs 31%, P = .23).
A phase II study by the Austrian Breast and Colorectal Cancer Study Group (ABCSG) evaluated exemestane in 80 postmenopausal women with ER+ and/or PgR+ operable breast cancer. After 4 months of treatment they found a clinical objective response rate of 34%, pCR rate of 3%, and BCS rate of 76%. These results are similar to those reported in randomized trials of nonsteroidal AIs.
A multicenter trial in the United States enrolled 150 (106 evaluable) postmenopausal women with ER+ or PgR+ breast cancer (>2 cm) to receive 16 to 24 weeks of preoperative letrozole 2.5 mg daily. The overall clinical response rate was 62%, which is comparable with the rate of 55% seen in the P024 trial. On multivariate analysis, several factors predicted for mastectomy rather than BCS: baseline T size (T3/4 vs T2), baseline surgical status (marginal candidate for breast conservation vs mastectomy/operable) and posttreatment clinical stage (T2–4 vs T1/0). Overall, 50% of the patients underwent BCS, including 30 of 46 who were initially marginal for BCS and 15 of 39 who were not candidates for BCS. In addition, all 11 patients who were initially felt to be inoperable were able to undergo surgical resection of their tumors. Nineteen percent of mastectomy patients were found to have a pathologic T1 tumor and therefore could have been candidates for BCS. The investigators concluded that these patients may have been upstaged with current preoperative imaging techniques, and that those patients who seem to have a good clinical response should have BCS attempted.
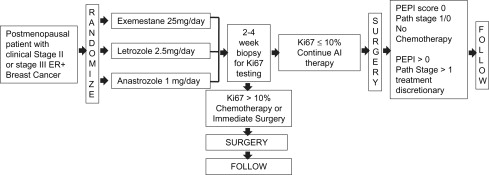
These trials demonstrate the benefit of AIs over tamoxifen in the neoadjuvant setting. A meta-analysis of 4 trials, the P024, IMPACT, PROACT, and the exemestane trials, was able to reconcile some of the variability in outcomes between the trials. The pooled analysis found a statistically significant improvement in the clinical objective response rate, the ultrasound objective response rate, and the BCS rate with the AIs compared with tamoxifen. A randomized trial (ACOSOG Z1031) comparing 3 AIs directly is underway, but results have not yet been reported ( Fig. 1 ).
AIs versus tamoxifen
AIs have been found to be superior to tamoxifen as endocrine therapy for postmenopausal women with hormone receptor–positive breast cancer in the settings of metastatic disease, locally advanced disease, and early-stage disease. The molecular mechanism explaining the benefit of AIs compared with tamoxifen is still not clear, but may be related to the difference in the mechanism of action between the 2 classes of drugs or pharmacogenomic variables that differentially affect drug metabolism, or both. For example, AIs block the conversion of androgens to estrogens in the peripheral tissues, thereby depriving the ER of its agonist. On the other hand, tamoxifen directly binds to the ER and acts as an agonist or antagonist in various tissues. Although the CYP2D6 genotype may be implicated in the efficacy of tamoxifen in some studies, the debate continues and germ line polymorphisms that affect AI efficacy are still being explored. Regardless, the improved outcomes with AIs compared with tamoxifen in postmenopausal women with ER+ breast cancer prompted several trials to be designed to compare these agents in the neoadjuvant setting.
One of the first trials to compare an AI with tamoxifen in the neoadjuvant setting was the P024 trial. This was a randomized, double-blind, multicenter study that randomized 324 postmenopausal women with ER+ and/or progesterone receptor (PgR)-positive breast cancer to letrozole 2.5 mg daily or tamoxifen 20 mg daily for 4 months. At enrollment none of the patients were felt to be candidates for breast-conserving surgery (BCS) and 14% of the patients were inoperable. In the intention-to-treat analysis there was a statistically significant improvement in the overall objective response rate (by clinical palpation) in the letrozole group compared with the tamoxifen group (55% vs 36%; P <.001). All secondary end points were also found to be significant between the 2 groups: ultrasound response, 35% versus 25% ( P = .042), mammographic response, 34% versus 16% ( P <.001), and BCS, 45% versus 35% ( P = .022). Few pathologic complete responses (pCR) were seen in the primary breast lesions in either group with only 2 in the letrozole group and 3 in the tamoxifen group.
The Pre-Operative Arimidex Compared to Tamoxifen (PROACT) trial compared anastrozole with tamoxifen in postmenopausal women with ER+ and/or PgR+ operable or potentially operable invasive breast cancer. This was a randomized, double-blind, double-dummy, multicenter study that randomized 451 patients to 3 months of anastrozole 1 mg daily or tamoxifen 20 mg daily. No significant difference in response rates was observed in the 137 patients who received concomitant neoadjuvant chemotherapy. After excluding those patients who received chemotherapy, similar response rates were seen in the anastrozole (36.2%) and tamoxifen (26.5%) groups ( P = .07). The objective response rates for anastrozole and tamoxifen were 48.6% and 35.8% (caliper-measured; P = .04), respectively, and 36.6% and 24.2% (ultrasound-measured; P = .03) in patients who were not eligible for BCS or were inoperable at baseline. Improvement in actual surgery occurred in 43% of anastrozole-treated patients and 31% of tamoxifen-treated patients ( P = .04).
Another neoadjuvant endocrine therapy trial, the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) trial, was a phase III randomized, double-blind, double-dummy, multicenter trial that randomized patients to anastrozole 1 mg and tamoxifen placebo daily, tamoxifen 20 mg and anastrozole placebo daily, or a combination of tamoxifen 20 mg and anastrozole 1 mg daily for 12 weeks before surgery. There were 330 postmenopausal women with invasive ER+, nonmetastatic, and operable or locally advanced potentially operable breast cancer included in the study. Objective response rates were 37%, 36%, and 39%, respectively, based on caliper measurement and 24%, 20%, and 28%, respectively, on ultrasound. These differences were not statistically significant. Of the patients felt to be candidates for BCS by their surgeons, a statistically significant number received anastrozole compared with tamoxifen (46% vs 22%, P = .03). There was a trend toward improved numbers of BCS in patients in the anastrozole arm compared with the tamoxifen arm, although this was not significant (44% vs 31%, P = .23).
A phase II study by the Austrian Breast and Colorectal Cancer Study Group (ABCSG) evaluated exemestane in 80 postmenopausal women with ER+ and/or PgR+ operable breast cancer. After 4 months of treatment they found a clinical objective response rate of 34%, pCR rate of 3%, and BCS rate of 76%. These results are similar to those reported in randomized trials of nonsteroidal AIs.
A multicenter trial in the United States enrolled 150 (106 evaluable) postmenopausal women with ER+ or PgR+ breast cancer (>2 cm) to receive 16 to 24 weeks of preoperative letrozole 2.5 mg daily. The overall clinical response rate was 62%, which is comparable with the rate of 55% seen in the P024 trial. On multivariate analysis, several factors predicted for mastectomy rather than BCS: baseline T size (T3/4 vs T2), baseline surgical status (marginal candidate for breast conservation vs mastectomy/operable) and posttreatment clinical stage (T2–4 vs T1/0). Overall, 50% of the patients underwent BCS, including 30 of 46 who were initially marginal for BCS and 15 of 39 who were not candidates for BCS. In addition, all 11 patients who were initially felt to be inoperable were able to undergo surgical resection of their tumors. Nineteen percent of mastectomy patients were found to have a pathologic T1 tumor and therefore could have been candidates for BCS. The investigators concluded that these patients may have been upstaged with current preoperative imaging techniques, and that those patients who seem to have a good clinical response should have BCS attempted.
These trials demonstrate the benefit of AIs over tamoxifen in the neoadjuvant setting. A meta-analysis of 4 trials, the P024, IMPACT, PROACT, and the exemestane trials, was able to reconcile some of the variability in outcomes between the trials. The pooled analysis found a statistically significant improvement in the clinical objective response rate, the ultrasound objective response rate, and the BCS rate with the AIs compared with tamoxifen. A randomized trial (ACOSOG Z1031) comparing 3 AIs directly is underway, but results have not yet been reported ( Fig. 1 ).
Neoadjuvant chemotherapy versus endocrine therapy
It is well known that adjuvant endocrine therapy is the most effective adjuvant treatment of ER+ breast cancer in postmenopausal women. Several studies examining the gene signatures of ER+ breast tumors have shown that there is a subgroup of patients that gain little from chemotherapy. Therefore, patients who are not responsive to chemotherapy in the adjuvant setting would not likely be responsive to chemotherapy in the neoadjuvant setting. Yet, there has been concern about the use of neoadjuvant endocrine therapy because of the low pCR rates seen in the neoadjuvant endocrine studies (<5%). However, in ER+ patients receiving neoadjuvant chemotherapy, there is also a low pCR rate. In a retrospective trial of 372 patients with locally advanced breast cancer who received 4 cycles of neoadjuvant doxorubicin-containing chemotherapy, there was a significant correlation between ER status and pCR rate. ER− tumors and those with a higher nuclear grade were more likely to have a pCR of the primary tumor and axillary lymph nodes that was independent of the initial tumor size ( P <.01). There was a significant difference in the pCR rate for ER+ tumors compared with ER− tumors (3% vs 17%; P <.01) demonstrating the ineffectiveness of chemotherapy in ER+ patients. Another retrospective study evaluating 435 patients who received neoadjuvant anthracycline or non–anthracycline-containing regimens correlated pCR rate and overall survival with ER status. They also found a lower pCR rate in ER+ tumors compared with ER− tumors (8.1% vs 21.6%; P <.001). Although there was improved overall survival in all patients who achieved a pCR in this study, the difference was not significant in ER+ patients who achieved a pCR compared with those who did not (93% vs 79%; P = .3). These studies all highlight that ER+ tumors are less responsive to chemotherapy, and therefore may need to be approached differently when planning neoadjuvant treatment.
The only study comparing neoadjuvant endocrine therapy to chemotherapy was a phase II study that randomized 239 postmenopausal women with ER+ and/or PgR+ breast cancer to endocrine therapy with either anastrozole 1 mg daily or exemestane 25 mg daily for 3 months or to chemotherapy with doxorubicin 60 mg/m 2 and paclitaxel 200mg/m 2 every 3 weeks for 4 cycles. All patients were felt to be ineligible for BCS initially and had T2N1-2, T3N0-1, or T4N0M0 disease. They found no statistically significant difference in the primary end point, overall objective response rate (by palpation), for endocrine therapy (anastrozole, 62%; exemestane, 67%) compared with chemotherapy (63%, P >.5). The pCR rates were not statistically different between the hormonal and chemotherapy groups (3% vs 6%; P >.05). The responses as determined by mammography and ultrasound were not statistically different between the hormonal and chemotherapy groups (60% vs 63%; P >.5 and 40% vs 46%; P >.5). In addition, there was a trend toward higher rates of BCS in the endocrine therapy group (33% vs 24%; P = .58). There was also a trend toward higher objective response rates and BCS among patients with strongly ER+ tumors (defined as Allred score ≥6) in the endocrine therapy group compared with the chemotherapy group (43% vs 24%; P = .054). This study at least demonstrates a similar efficacy of neoadjuvant endocrine therapy and chemotherapy in postmenopausal women with ER-expressing tumors. In addition, it suggests that there may be improved responses and BCS rates in patients receiving endocrine therapy compared with chemotherapy. More randomized studies are necessary to clarify this further.
Biomarker evaluation in the neoadjuvant endocrine setting to predict long-term outcome
Neoadjuvant chemotherapy studies have used pCR as a means to demonstrate response to a regimen. However, this end point is less useful in patients with ER+, human epidermal growth factor receptor 2 (HER2)− disease treated with endocrine therapy because pCRs are infrequently seen and do not necessarily correlate with long-term outcome. Expression of the proliferative antigen Ki67 after a short-term of drug exposure has been proposed in assessing tumor response to endocrine therapy in the neoadjuvant setting. This marker may be suppressed after only a short duration of treatment, thereby making it a tool to predict which patients are most likely to respond to further endocrine treatment.
In the P024 trial, pretreatment biopsies were compared with surgical specimens after 4 months of treatment with either letrozole or tamoxifen. Letrozole was found to produce greater suppression of Ki67 compared with tamoxifen (87% inhibition of geometric mean Ki67 vs 75%; P = .0009). This decrease in Ki67 was found to be significantly greater in responders than nonresponders ( P = .025). Similarly in the IMPACT study, biopsies taken at 2 and 12 weeks showed a significantly greater mean suppression of Ki67 with anastrozole (76% and 81.6%) than with tamoxifen (59.5% and 61.9%). Unlike the P024 trial, a significant correlation between the degree of Ki67 suppression and clinical response was not found. A trend toward greater suppression of Ki67 at 2 and 12 weeks in patients downstaged from mastectomy to BCS was evident. However, not all tumors that respond to endocrine therapy have significant decreases in Ki67, and tumors with suppression of Ki67 do not all have significant responses.
To identify other posttreatment factors that might predict breast cancer survival after neoadjuvant endocrine therapy, a multivariate analysis was performed on the P024 trial. Four factors were found to have independent prognostic value for relapse-free survival and breast cancer–specific survival: pathologic tumor size (T1/2 vs T3/4), pathologic node status (positive or negative), and 2 biomarkers in the surgical resection specimen, the normal log of the Ki67 value and the ER status of the tumor. A prognostic score, the preoperative endocrine prognostic index (PEPI), was developed, which weighs each of these factors according to the magnitude of their hazard ratios ( Table 1 ). Three risk groups were formed (score 0, 1–3, and ≥4) that were associated with relapse risks of 10%, 23%, and 48% ( P <.001). For breast cancer death the corresponding risks were 2%, 11%, and 17% ( P <.001). The PEPI was then validated in an independent data set from the IMPACT trial. In both trials relapses were not seen in patients with pathologic stage 0 or 1 disease with a PEPI score of 0. The PEPI score has become a useful tool to identify patients who are sensitive to estrogen inhibition that integrates multiple biologic variables.





