Neoadjuvant chemotherapy (NAC) is being used with increasing frequency in the multidisciplinary care of women with breast cancer. NAC can increase the chances for successful breast conservation and may decrease the need for axillary node dissection in selected patients. Some patients with chemoresistant tumors may benefit more from hormonal neoadjuvant therapy. The greatest potential for this approach is to predict responses of different breast cancers to therapy based on molecular profiles. This will accelerate better understanding of breast cancer biology and progress toward improved and more individualized therapy.
Potential benefits of neoadjuvant chemotherapy for breast cancer
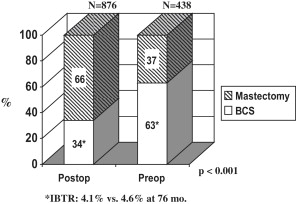
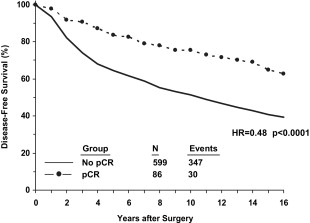
Classically, neoadjuvant chemotherapy (NAC) was used only for patients with locally advanced breast cancer (LABC), corresponding approximately to American Joint Commission on Cancer stage III. Once the success of downstaging those patients with chemotherapy was appreciated, similar strategies began to be used for patients with operable breast cancer but who were not ideal candidates for breast-conserving surgery (BCS). Initially, this approach was tested to determine whether primary or neoadjuvant chemotherapy might prove superior to postoperative or adjuvant therapy, but it was hoped that this also might allow BCS for women who would otherwise require mastectomy. As shown in the National Surgical Adjuvant Breast and Bowel Project’s (NSABP) Protocol B-18, the former was not the case, but the latter was feasible. In this trial, women with palpable operable breast cancer (median tumor size = 3.5 cm) diagnosed by needle biopsy were randomized to receive 4 cycles of doxorubicin (Adriamycin) + cyclophosphamide (AC) preoperatively or the same regimen postoperatively in the adjuvant setting. The breast conservation rates were 68% and 60% for the 2 groups, respectively, and the overall outcomes (disease-free survival [DFS] and overall survival [OS]) for both groups of patients were identical. These data showed that NAC could be used to increase the likelihood of BCS without compromising survival. The increase in BCS was even more striking in the European Cooperative Trial in Operable Breast Cancer (ECTO) trial, as shown in Fig. 1 . The BCS rate for primary surgery patients in the European study was lower than in B-18, which may reflect a greater frequency of larger tumors (80% >4 cm), as well as possible systematic differences in surgical practice. Nevertheless, other studies have confirmed the potential to increase BCS, as well as the potential to decrease the volume of tissue that needs to be removed and the need for re-excision. The NSABP B-18 study also demonstrated a strong relationship between pathologic complete response (pCR, defined in B-18 as no invasive breast cancer cells in the breast) with NAC and patient outcomes ( Fig. 2 ), which has been observed repeatedly in other series as well. It has been suggested that the ability to observe responses during neoadjuvant chemotherapy treatment constitutes another potential advantage to this approach, but it is not clear how this information should be used to modify the treatment of each patient in real time. A German study designed to determine whether an early change in chemotherapy drugs would benefit patients who do not respond well to the initial regimen showed that nonresponders are unlikely to experience a pCR with either more of the same or a different cytotoxic regimen. Nevertheless, even without a pCR, continuing chemotherapy may reduce tumor burden sufficiently to make inoperable cancers operable or to allow BCS. Moreover, it may be beneficial for patients to see the effects of treatment as the tumors regress, in contrast to the adjuvant setting where the benefits are hypothetical and the toxicities extremely concrete. Another advantage, albeit controversial, to neoadjuvant chemotherapy is the potential to downstage the axillary nodes and decrease the need for axillary node dissection and the risk of lymphedema. This aspect of surgical management after NAC is discussed in more detail later. The potential clinical advantages of NAC for breast cancer are
- 1.
Increased chance for BCT
- 2.
Improved cosmesis by removal of less tissue and fewer re-excisions
- 3.
Decreased need for axillary node dissection
- 4.
Time for genetic testing before surgery
- 5.
Observation of response to treatment
- 6.
Opportunity for discovery.
Because of the strong relationship of response (especially pCR) to patient outcomes, NAC offers a valuable research platform to test new treatment regimens and agents, and to increase our understanding of breast cancer biology; these in turn will lead to truly individualized therapy based on biologic and molecular parameters.
Predicting response to neoadjuvant chemotherapy
Based on these advantages, it has been suggested that anyone with a T2 or larger tumor or clinically evident or ultrasound (US)-detected lymph node involvement at presentation is a potential candidate for NAC. A less well-defined, but perhaps more practical, guideline might be that NAC should be considered for any patient in whom it is clear that adjuvant chemotherapy would be indicated. As more is learnt about breast cancer biology, this decision will be based only partly on the anatomic clinical stage and more on the pathologic and molecular features of each cancer.
Indeed, as NAC has become more widely applied, it has become evident that some patients may benefit more from NAC than others. At the simplest level, multiple studies, including the NSABP B-27 and ECTO trials, have shown that hormone-responsive breast cancers are less likely to achieve pCR than hormone receptor negative cancers. In the B-27 trial, for example, estrogen receptor (ER)-positive cancers had a pCR rate of 8.3% versus 16.7% for ER-negative tumors. However, both subsets of patients appeared to benefit, in terms of pCR, from the addition of a taxane to the neoadjuvant regimen. In the ECTO trial, ER was a highly significant predictor of pCR (12% for ER+ vs 42% for ER−). Despite the emphasis on pCR in the literature, many patients with ER+ cancers may still benefit substantially from NAC. In the B-18 trial, 80% of all patients experienced an objective clinical response to AC, and in B-27 more than 90% of patients treated with AC + docetaxel had an objective response. In the ECTO trial, 42% of patients with ER+ tumors had a clinical complete response (cCR) compared with 60% in the ER− subset. The MD Anderson experience shows that pCR is strongly predictive for outcomes in the ER+ subset. Thus, a predictably low likelihood of a pCR does not mean that NAC would not be clinically useful, perhaps allowing BCS in many ER− and ER+ patients who would otherwise require a mastectomy. What might be more helpful, and could perhaps be provided by the more detailed molecular characterizations to be discussed later, would be the ability to predict which patients are unlikely to have an objective and clinically useful response to chemotherapy. These patients would then be candidates for immediate surgery, neoadjuvant hormonal therapy, or neoadjuvant treatment with novel agents.
Patients with invasive lobular cancers (ILC) have also been singled out in multiple studies as a subset who are less likely to achieve a pCR. In some studies, the pCR rate for these patients has been less than 5%, but in the in B-27 trial 10.2% of ILC patients (vs 17.5% for IDC) had a pCR. Moreover, it has recently been suggested that NAC may not dramatically increase the likelihood of BCS in patients with lobular cancers, making this approach less advantageous. Despite the low response rates compared with IDC, patients with ILC generally have better long-term outcomes. The low chemosensitivity of ILC likely reflects the strong hormone responsiveness for most of these tumors, and the better outcomes reflect a less aggressive biology. This group of patients may benefit more from hormonal neoadjuvant therapy than from cytotoxic chemotherapy.
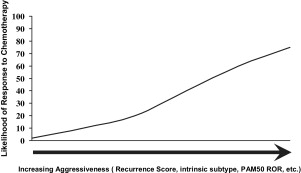
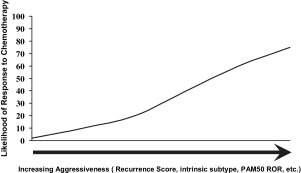
A clinical and histopathologic scoring system to predict the likelihood of responding well to NAC has been described. Recently, molecular/genetic profiles of tumors have been shown to be potentially more precise predictors of who will or will not respond to NAC for breast cancer. Gianni and colleagues showed that the 21-gene expression profile originally developed to predict outcomes for patients with node-negative ER+ cancers was highly predictive of pCR to NAC. Only patients with high recurrence scores exhibited pCR to polychemotherapy. Using clinical response as an end point, it has also been shown that recurrence score could identify patients who were most likely to have a clinical complete response to single-agent taxane therapy. Many other studies have shown that some gene expression profiles that correlate with a poor prognosis (eg, HER-2 enriched and basal-like) also correlate with a high likelihood of a good response to NAC ( Fig. 3 ; Table 1 ). Conversely, these data show that tumors with profiles indicating less aggressive behavior are also less likely to respond to chemotherapy, which is probably the major reason for the imperfect relationship between pCR and patient outcomes and may also explain why doubling the pCR rate with the addition of a taxane to AC in B-27 did not lead to a significant increase in survival. Because patients who do not have a pCR with NAC are a mixture of patients with nonaggressive tumors with a good prognosis (eg, the luminal A subset) and more aggressive tumors with a poor prognosis that are also chemoresistant, these patients have intermediate outcomes. If both subsets of chemoresistant patients could be clearly identified, the patients with good prognosis could avoid toxic therapies and the subset with poor prognosis would be candidates for trials of novel agents. A recent report combining patients from the B-27 trial, the I-SPY trial, and patients from MD Anderson, all of whom received anthracycline + taxane NAC, suggests that the luminal B subset responds poorly to chemotherapy and has a poor prognosis. As suggested earlier, rather than focusing on pCR, it may be more important to understand which patients may not benefit clinically from NAC (eg, by increasing the chance for BCS) or from adjuvant chemotherapy. Such patients may benefit more from hormonal therapy or new biologic agents and might avoid chemotherapy altogether. Although it is not yet standard to perform a molecular/genetic assessment of breast cancers from core needle biopsy material before making a decision about neoadjuvant treatment, we are certainly heading in that direction. One of the major goals of the current NSABP B-40 trial is to gather data that will inform that course.
| Study | (Method)/Subsets | % pCR |
|---|---|---|
| Esserman, ASCO 2009 |
| |
| 3 | |
| Intermediate RS | 0 | |
| High RS | 36 | |
| Parker, SABCS 2009 | (PAM 50) | |
| Luminal A | 7 | |
| Luminal B | 17 | |
| HER-2 | 36 | |
| Basal-like | 43 | |
| Normal-like | 19 | |
| Straver | (70-gene signature [Mammaprint]) | |
| Good signature | 0 | |
| Poor signature | 20 | |
| Chang, 2008 | 21-gene score | |
| Low RS | 0 a | |
| High RS | 21 a | |
| Liedtke, 2009 | 97-gene genomic grade index | |
| High risk | 12 b | |
| Low risk | 40 b |
a Clinical complete responses.
Predicting response to neoadjuvant chemotherapy
Based on these advantages, it has been suggested that anyone with a T2 or larger tumor or clinically evident or ultrasound (US)-detected lymph node involvement at presentation is a potential candidate for NAC. A less well-defined, but perhaps more practical, guideline might be that NAC should be considered for any patient in whom it is clear that adjuvant chemotherapy would be indicated. As more is learnt about breast cancer biology, this decision will be based only partly on the anatomic clinical stage and more on the pathologic and molecular features of each cancer.
Indeed, as NAC has become more widely applied, it has become evident that some patients may benefit more from NAC than others. At the simplest level, multiple studies, including the NSABP B-27 and ECTO trials, have shown that hormone-responsive breast cancers are less likely to achieve pCR than hormone receptor negative cancers. In the B-27 trial, for example, estrogen receptor (ER)-positive cancers had a pCR rate of 8.3% versus 16.7% for ER-negative tumors. However, both subsets of patients appeared to benefit, in terms of pCR, from the addition of a taxane to the neoadjuvant regimen. In the ECTO trial, ER was a highly significant predictor of pCR (12% for ER+ vs 42% for ER−). Despite the emphasis on pCR in the literature, many patients with ER+ cancers may still benefit substantially from NAC. In the B-18 trial, 80% of all patients experienced an objective clinical response to AC, and in B-27 more than 90% of patients treated with AC + docetaxel had an objective response. In the ECTO trial, 42% of patients with ER+ tumors had a clinical complete response (cCR) compared with 60% in the ER− subset. The MD Anderson experience shows that pCR is strongly predictive for outcomes in the ER+ subset. Thus, a predictably low likelihood of a pCR does not mean that NAC would not be clinically useful, perhaps allowing BCS in many ER− and ER+ patients who would otherwise require a mastectomy. What might be more helpful, and could perhaps be provided by the more detailed molecular characterizations to be discussed later, would be the ability to predict which patients are unlikely to have an objective and clinically useful response to chemotherapy. These patients would then be candidates for immediate surgery, neoadjuvant hormonal therapy, or neoadjuvant treatment with novel agents.
Patients with invasive lobular cancers (ILC) have also been singled out in multiple studies as a subset who are less likely to achieve a pCR. In some studies, the pCR rate for these patients has been less than 5%, but in the in B-27 trial 10.2% of ILC patients (vs 17.5% for IDC) had a pCR. Moreover, it has recently been suggested that NAC may not dramatically increase the likelihood of BCS in patients with lobular cancers, making this approach less advantageous. Despite the low response rates compared with IDC, patients with ILC generally have better long-term outcomes. The low chemosensitivity of ILC likely reflects the strong hormone responsiveness for most of these tumors, and the better outcomes reflect a less aggressive biology. This group of patients may benefit more from hormonal neoadjuvant therapy than from cytotoxic chemotherapy.
A clinical and histopathologic scoring system to predict the likelihood of responding well to NAC has been described. Recently, molecular/genetic profiles of tumors have been shown to be potentially more precise predictors of who will or will not respond to NAC for breast cancer. Gianni and colleagues showed that the 21-gene expression profile originally developed to predict outcomes for patients with node-negative ER+ cancers was highly predictive of pCR to NAC. Only patients with high recurrence scores exhibited pCR to polychemotherapy. Using clinical response as an end point, it has also been shown that recurrence score could identify patients who were most likely to have a clinical complete response to single-agent taxane therapy. Many other studies have shown that some gene expression profiles that correlate with a poor prognosis (eg, HER-2 enriched and basal-like) also correlate with a high likelihood of a good response to NAC ( Fig. 3 ; Table 1 ). Conversely, these data show that tumors with profiles indicating less aggressive behavior are also less likely to respond to chemotherapy, which is probably the major reason for the imperfect relationship between pCR and patient outcomes and may also explain why doubling the pCR rate with the addition of a taxane to AC in B-27 did not lead to a significant increase in survival. Because patients who do not have a pCR with NAC are a mixture of patients with nonaggressive tumors with a good prognosis (eg, the luminal A subset) and more aggressive tumors with a poor prognosis that are also chemoresistant, these patients have intermediate outcomes. If both subsets of chemoresistant patients could be clearly identified, the patients with good prognosis could avoid toxic therapies and the subset with poor prognosis would be candidates for trials of novel agents. A recent report combining patients from the B-27 trial, the I-SPY trial, and patients from MD Anderson, all of whom received anthracycline + taxane NAC, suggests that the luminal B subset responds poorly to chemotherapy and has a poor prognosis. As suggested earlier, rather than focusing on pCR, it may be more important to understand which patients may not benefit clinically from NAC (eg, by increasing the chance for BCS) or from adjuvant chemotherapy. Such patients may benefit more from hormonal therapy or new biologic agents and might avoid chemotherapy altogether. Although it is not yet standard to perform a molecular/genetic assessment of breast cancers from core needle biopsy material before making a decision about neoadjuvant treatment, we are certainly heading in that direction. One of the major goals of the current NSABP B-40 trial is to gather data that will inform that course.
| Study | (Method)/Subsets | % pCR |
|---|---|---|
| Esserman, ASCO 2009 |
| |
| 3 | |
| Intermediate RS | 0 | |
| High RS | 36 | |
| Parker, SABCS 2009 | (PAM 50) | |
| Luminal A | 7 | |
| Luminal B | 17 | |
| HER-2 | 36 | |
| Basal-like | 43 | |
| Normal-like | 19 | |
| Straver | (70-gene signature [Mammaprint]) | |
| Good signature | 0 | |
| Poor signature | 20 | |
| Chang, 2008 | 21-gene score | |
| Low RS | 0 a | |
| High RS | 21 a | |
| Liedtke, 2009 | 97-gene genomic grade index | |
| High risk | 12 b | |
| Low risk | 40 b |
a Clinical complete responses.
Chemotherapy versus hormonal therapy
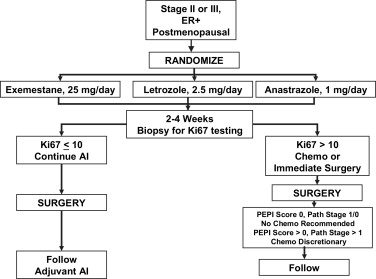
As noted earlier, there are emerging data suggesting that certain subsets of tumors, especially among those that are ER+ and/or PR+, may not respond well or in a clinically useful way to chemotherapy. For example, in the I-SPY trial, only 5% of luminal A tumors had a pCR to chemotherapy. As recently reviewed, aromatase inhibitors seem to be superior to tamoxifen as neoadjuvant therapy for postmenopausal women with hormone-responsive tumors. Aromatase inhibitors as neoajduvant treatment of such women have been shown to be at least as effective as, if not superior to, chemotherapy in terms of clinical responses and conversion to BCS. However, pCR rates are consistently low for hormonal neoadjuvant therapy. As increasingly sophisticated molecular predictors of response to neoadjuvant chemotherapy or hormonal therapy are developed, we will be better able to choose among these alternative approaches for individual patients. Recently it has been proposed that early molecular assessments of response to hormonal therapy based on re-biopsy after 2 to 4 weeks of treatment may be a useful tool for deciding whether to continue hormonal therapy or switch to chemotherapy. This concept is being tested directly in the ongoing American College of Surgeons Oncology Group (ACOSOG) Z1031B trial ( Fig. 4 ). There are also subsets of patients, based on age and comorbidities, who may not be appropriate candidates for cytotoxic chemotherapy, but who may benefit from neoadjuvant hormonal therapy.
Pre-therapy assessment and staging
Once the decision has been made to treat a patient with breast cancer with NAC, several studies in addition to those that are routine (mammograms, routine laboratory tests, and so forth) should be performed. Although controversial as a standard study for all breast cancers, magnetic resonance imaging (MRI) of the breasts is particularly valuable for NAC patients. MRI provides information about the extent of the known cancer, possible multicentric disease, axillary and internal mammary nodes, and serves as a baseline for evaluation of the primary tumor and nodal response to treatment. It has also been suggested that the appearance of the breast primary on MRI may be predictive of the likelihood of response to NAC. For patients with LABC (stage III), evaluation for metastatic disease would be appropriate. It is also valuable to examine the axilla by US and to perform a biopsy on any suspicious-appearing nodes before starting treatment. This will provide important information that may influence lymph node surgery and/or radiation decisions later.
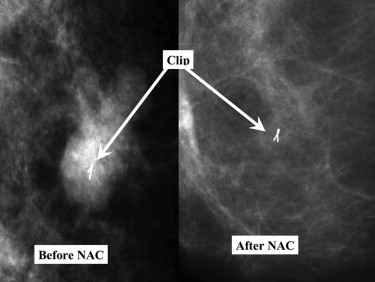
If the patient is being considered for BCS, it is critically important that the primary tumor site be tagged so that the appropriate area is excised at definitive surgery. At the completion of therapy, many palpable tumors will no longer be clinically or even mammographically detectable ( Fig. 5 ), and the clip can be targeted preoperatively with a localizing wire or by intraoperative US. In some centers, a radiographically guided clip is placed only if the tumor shows evidence of rapid regression, but it is our routine to place a clip in all of these tumors before starting therapy to avoid missing the opportunity to do so mid-therapy. Placement of clips in patients receiving NAC has even been shown to improve local control after BCS. Even if a total mastectomy is planned at the time of presentation, the clip can be helpful to the pathologist who examines the resection specimen, because the primary tumor site may become difficult to identify grossly. Moreover, some patients and/or their surgeons may change their minds about the surgical plan. In addition, we routinely tattoo the skin overlying the margins of the palpable tumor mass in the breast (usually at the time of venous access placement in the operating room), even if the patient has had a clip placed in the tumor ( Fig. 6 ). This marking is a valuable aid to the ongoing clinical evaluation of the tumor response during therapy and helps to plan surgery, often making wire localization of the tumor site unnecessary.