Sublobar resections are a treatment modality primarily used for early-stage non-small cell lung cancer (NSCLC) in patients with limited pulmonary reserve, where a larger anatomic resection would not be tolerated. Sublobar resections are also currently under prospective randomized evaluation in two independent trials as an alternative treatment strategy for healthy patients with small, peripheral, node-negative tumors. Lobectomy is currently the recommended treatment for stage I and II NSCLC according to the American College of Chest Physicians (ACCP) evidence-based guidelines for the diagnosis and management of lung cancer
1 and the National Comprehensive Cancer Network (NCCN) guidelines.
2 These recommendations are based primarily on results from the only randomized trial completed to date which compared lobectomy to sublobar resection for stage I NSCLC and noted a threefold increase in local recurrence and trend toward worse survival in those undergoing sublobar resection.
3A sublobar resection for NSCLC is defined as either an anatomic segmentectomy or a wide wedge resection. Segmentectomy is generally considered to be the oncologically superior of the two approaches, typically with wider margins and greater numbers of harvested lymph nodes than wedge resection.
4 Segmentectomy is therefore the preferred sublobar approach for primary resection of NSCLC when technically and medically
feasible. A systematic evaluation of the mediastinal lymph nodes is recommended for all sublobar resections performed for NSCLC.
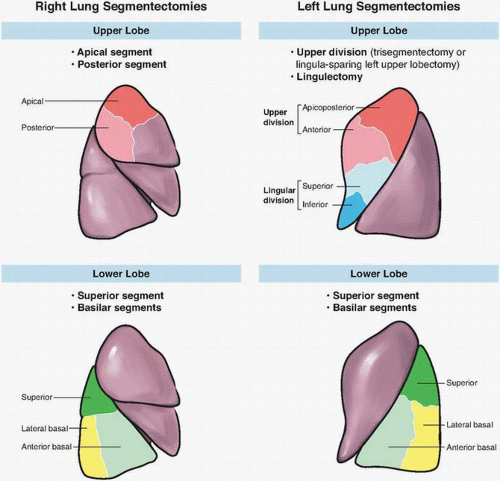
Surgeons undertaking segmentectomy need to be familiar with segmental anatomy. There are 19 pulmonary segments, 10 on the right and 9 on the left, with the anterior basal and medial basal on the left considered a single segment. Although segmental resections have been described for nearly all bronchial segments, generally accepted segmental resections are outlined in
Figure 10-1.
5,
6 Suggested criteria for use of segmentectomy include small tumors (<3 cm), location in the peripheral one-third of the lung, and no endobronchial involvement. Such conditions allow for adequate surgical margins.
7,
8 Careful review of the chest computed tomography (CT) is suggested preoperatively. This allows the surgeon to ascertain if the tumor is confined within the segmental boundary and appropriate for sublobar techniques.
5,
6,
9,
10 It is also helpful for determining the adequacy of margins and identification of aberrant anatomy. Flexible bronchoscopy is indicated to ensure absence of endobronchial disease and delineating bronchial anatomy prior to division.
10 General anesthesia with single lung ventilation is beneficial to maintain a quiet operative field for open
approaches and is mandatory to obtain the pneumothorax required for minimally invasive approaches. Lateral decubitus positioning with flexion is recommended. Thoracotomy, video-assisted thoracoscopic surgery (VATS), or robotic-assisted approaches are all acceptable for sublobar resections, and the approach should not alter the technical aspects of the procedure.
6 The optimal approach depends on the patient and tumor characteristics and the surgeon’s expertise. The efficacy of minimally invasive techniques for wedge resection has been long accepted.
11 Numerous nonrandomized trials and single institution case series have demonstrated equivalent short- and long-term oncologic outcome for segmental resections by VATS and open techniques.
12,
13,
14,
15,
16,
17,
18,
19 Although no prospective randomized trials comparing the different approaches have been completed, limited evidence suggests VATS may be better tolerated with reduced pain and length of stay and greater postoperative independence.
19,
20,
21,
22,
23,
24 The greatest benefit from minimally invasive approaches appears to be in those patients with severe medical comorbidity. Data from the Society of Thoracic Surgery General Thoracic Surgery Database suggest significant reductions in perioperative morbidity and mortality with minimally invasive approaches to lung resections in high-risk population.
25,
26 The robotic approach is the most novel and has the least evidence for efficacy for segmental resections, but a recent series reports safety and feasibility for the approach.
27