Salivary Glands
MAJOR SALIVARY GLANDS
Anatomy
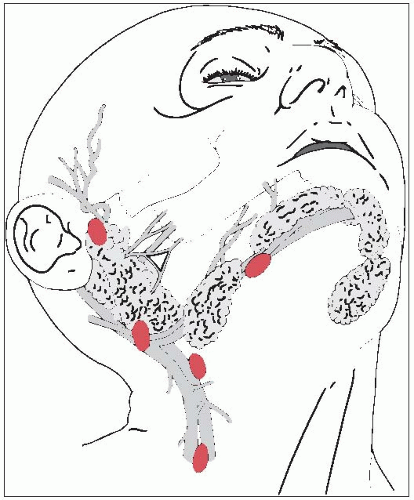
The salivary glands consist of three large, paired major glands (parotid, submandibular, and sublingual) and many smaller, minor glands located throughout the upper aerodigestive tract (Fig. 16-1).
Parotid Gland
The parotid, the largest of the three salivary glands, is located superficial to and partly behind the ramus of the mandible and covers the masseter muscle.
Superficially, the parotid overlaps the posterior part of the muscle and largely fills the space between the ramus of the mandible and the anterior border of the sternocleidomastoid muscle.
The facial nerve enters the deep surface of the gland as a single trunk, passing posterolaterally to the styloid process. Removal of all or part of the parotid gland demands meticulous dissection if the nerve is to be spared.
Lymphatics drain laterally on the face, including parts of the eyelids, diagonally downward and posteriorly toward the parotid gland, as do the lymphatics from the frontal region of the scalp.
Submandibular Gland
The submandibular gland largely fills the triangle between the two bellies of the digastric and the lower border of the mandible and extends upward deeply to the mandible.
It lies partly on the lower surface of the mylohyoid and partly behind the muscle against the lateral surface of the muscle of the tongue, the hypoglossus.
Bimanual palpation with one finger in the floor of the mouth and one under the edge of the mandible facilitates clinical detection of masses in this gland.
A fairly rich lymphatic capillary network lies in the interstitial spaces of the gland and drains to submandibular or subdigastric nodes.
Sublingual Gland
The smallest of the three major salivary glands, the sublingual gland, along with many minor salivary glands, lies between the mucous membrane of the floor of the mouth above, the mylohyoid muscle below, the mandible laterally, and the genioglossus muscles of the tongue medially.
This is a rare site for malignant neoplasms, which often are combined with minor salivary gland tumors originating in the floor of mouth.
The sublingual gland drains either to the submandibular lymph nodes or, more posteriorly, into the deep internal jugular chain.
Natural History
Up to 33% of tumors that arise in the submandibular gland invade the lower jaw.
As many as 25% of patients with malignant parotid tumors present with lymph node metastases.
High-grade tumors, regardless of histologic type, have a high (49%) risk of occult lymph node metastasis, compared with only a 7% risk for intermediate- or low-grade tumors (2).
Patients with epidermoid cancer have a particularly high risk of occult metastases, although metastatic squamous cell cancer of the skin must be excluded as a potential primary source.
Lymph node involvement at presentation is common (44%) with submandibular gland malignancies (2).
Clinical Presentation
Patients most often have a painless, rapidly enlarging mass, which is often present for years before a sudden change in its indolent growth pattern prompts the patient to seek medical attention.
Although as many as 25% of patients with parotid cancers have facial nerve involvement, only 10% complain of pain.
Diagnostic Workup
The diagnostic workup of major salivary gland tumors includes a careful history and physical examination, with particular attention to signs of local fixation or regional adenopathy.
Computed tomography is useful in evaluating the extent of lesions involving the parotid gland, especially the deep lobe.
Magnetic resonance imaging provides excellent anatomic detail.
Because of the heterogeneity of malignant salivary gland tumors, an open biopsy technique is used if a malignant diagnosis is anticipated; definitive surgery is performed if the diagnosis is confirmed.
Staging System
The American Joint Committee staging system for major (parotid, submandibular, and sublin gual) salivary gland sites is based on size, extension, and nodal involvement (1) (Table 16-1).
Pathologic Classification
Most parotid masses are benign. Only 21% of 231 parotid masses seen at Washington University, St. Louis, Missouri, were malignant (3).
Among atomic bomb survivors of Hiroshima, the relative risk for malignant parotid tumors in those exposed was 9.8, compared to the nonexposed population; the relative risk for minor salivary gland tumors among those exposed was 12.3 (p < 0.005) (22).
The most common malignant subtype of parotid tumors in children is the mucoepidermoid tumor, accounting for almost 50% of cases (4). Fifty-seven percent of parotid gland tumors in children are malignant, compared with only 15% to 25% in adults (4). A predominance of this cell type also occurs in adult parotid cancer (21).
Acinic cell carcinoma usually occurs only in the parotid gland (5).
In the submaxillary gland and in minor salivary glands, adenoid cystic carcinoma is the most common cancer.
Prognostic Factors
Survival is influenced most by tumor grade, postsurgical residual disease, tumor size, facial nerve invasion, and presence of positive cervical nodes. Overexpression of p53 and HER-2/neu, as well as aneuploidy, has been associated with poor prognosis.
General Management
General management in most patients includes surgical excision followed by radiation therapy for high-risk prognostic factors.
Low-grade tumors of the parotid are usually treated with a superficial parotidectomy, unless the lesion begins in the deep lobe.
A neck dissection is not electively done for low-grade tumors.
Surgical treatment includes neck dissection in patients with clinically positive nodes or high-grade, high-stage disease.
If the facial nerve is not involved by tumor, a nerve-sparing operation is generally done. If the facial nerve is involved, reconstruction of the facial nerve trunk by a cable nerve graft with the sural or greater auricular nerve decreases the incidence of postoperative facial palsy.
Adjuvant chemotherapy is not efficacious and should be reserved for the palliative setting.
Postoperative irradiation is indicated for microscopic or macroscopic residual disease, recurrent cancer, intermediate- and high-grade tumors, all adenoid cystic carcinomas, neural or perineural invasion, lymph node metastases, lymphatic or vascular invasion, and T3 to T4 malignancies.
The efficacy of radiation therapy for residual disease after surgery was illustrated in a subset analysis of 35 patients who had microscopic tumor at or close to margins after curative surgery. Only 3 of 22 patients (14%) who received postoperative irradiation had local recurrences, whereas 7 of 13 nonirradiated patients (54%) had local recurrences (p < 0.05) (9).
A local control rate of 87% was reported for parotid cancers treated with postoperative irradiation (15).
Definitive radiation therapy is indicated for medically inoperable or unresectable cancers.
High linear energy transfer (LET) radiation, such as neutron therapy, has been advocated for advanced and recurrent neoplasms, particularly of the parotid gland. Local control rates of 67% for major and 50% for minor salivary gland tumors have been achieved (19).The adjuvant setting in which neutron therapy is most likely to be advantageous over photon therapy is in patients with gross residual disease (8).
A small randomized trial of photon versus neutron therapy in inoperable parotid carcinoma suggested that neutron provided superior locoregional control (11).
Review of world literature found a locoregional control rate of 67% for fast neutrons but only 25% for photons or electrons in the treatment of inoperable, unresectable, or recurrent disease (10).
TABLE 16-1American Joint Committee Staging System for Major Salivary Gland Cancer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||