Chapter 35 Salivary Gland Malignancies
Etiology and Epidemiology
The causes of salivary gland tumors, both benign and malignant, have not been clearly established. Reports consistently suggest etiologic associations with nutritional deficiencies, exposure to ionizing radiation, ultraviolet light exposure, genetic predisposition, history of previous cancer of the skin of the face, occupational exposure, viral (e.g., Epstein-Barr virus) infection, alcohol use, hair dye use, and higher educational attainment.1,2,3,4,5
Of these associations, exposure to ionizing radiation may be regarded as the one established risk factor for salivary gland tumors, both benign and malignant. Studies have demonstrated not only a strong association6 but also a temporal latency (10 to 25 years) and a dose-response relationship with the exposure, especially for mucoepidermoid carcinomas.4,7,8 The risk is greater for malignant than benign salivary gland tumors.4,8 These observations are based on several independent long-term cohort studies of victims of atomic bomb exposure6,7,9 and patients receiving therapeutic radiation for benign8 and malignant indications.4 Radiation-induced malignant salivary gland tumors may occur with a higher frequency in the minor salivary glands.
Salivary gland tumors are regarded as rare, with a reported overall incidence in the Western world of approximately 2.5 cases to 3.0 cases per 100,000 per year.10 Malignant salivary gland tumors account for more than 0.5% of all malignancies and 3% to 5% of all head and neck cancers.10 They comprise approximately 11% of all oropharyngeal cancers.11 Most patients with malignant salivary gland tumors are in the sixth or seventh decade of life.12
The incidence patterns of salivary gland cancers were recently reported in a Surveillance, Epidemiology, and End Results (SEER) population-based study that offers the advantage of eliminating the inherent biases of clinical series.13 Almost 6,400 major salivary gland carcinomas were estimated to have been diagnosed from 1992 to 2006. The most common nonsquamous salivary gland cancers were mucoepidermoid carcinomas, followed by adenoid cystic and acinic carcinomas with comparable incidence rates. Males had a 51% higher incidence rate compared with females. The parotid gland accounted for 80% of all major salivary gland malignancies,13 but tumors of the parotid are in general less likely to be malignant compared with the other major salivary glands and the palate of the oral cavity.10 Except for adenoid cystic carcinomas, which were equally seen in the parotid and submandibular glands, other histologies occurred mainly in the parotid gland.
Among males, the most common nonsquamous salivary gland cancers were mucoepidermoid carcinoma and adenocarcinoma not otherwise specified (NOS).13 Among females, the most common nonsquamous salivary gland cancers were mucoepidermoid, acinic cell, and adenoid cystic carcinomas. Squamous cell carcinoma, adenocarcinoma NOS, and salivary duct carcinoma occurred with more than a twofold higher incidence among males. In contrast, females had a higher incidence of acinic cell and adenoid cystic carcinomas.
Acinic cell carcinomas were more likely to be diagnosed at younger ages compared with other cell types.13 Incidence ratios of salivary gland cancers were significantly lower among African Americans, Asians, and Pacific Islanders compared with whites. However, incidences of mucoepidermoid carcinoma and adenoid cystic carcinoma occurred equally among all races.
Prevention and Early Detection
The recognition that radiation exposure is a risk factor for benign and malignant salivary gland tumors (especially mucoepidermoid carcinomas) argues that long-term surveillance is likely a prudent recommendation but with unproven benefit. Patients with a known history of accidental, diagnostic, or therapeutic radiation head and neck exposure should be counseled about the potential risk of late manifestation of malignant tumors, especially if their anticipated life span may be 10 to 25 years. It is not clear if younger patients receiving therapeutic radiation are at an increased risk,4,8 necessitating more vigilant observation. Issues of cost-effectiveness of various surveillance investigations and the frequency of follow-up have not been established.
Because a dose-response relationship for developing salivary gland tumors from therapeutic radiation has been described (especially for mucoepidermoid carcinomas), this raises the intriguing question of whether this risk may be mitigated with modern radiation treatment planning techniques. Modern radiotherapy techniques have the advantage that the dose and volume of normal tissue irradiated may be reduced. This includes the use of proton radiation with its favorable Bragg peak physical beam characteristic. Details of this issue are further discussed in the section considering the role of protons in the management of salivary gland tumors. At this time this possibility remains largely speculative because the risk of developing salivary gland tumors has been demonstrated even with low doses (i.e., doses less than 280 cGy to the parotid glands).8 Thus the judicious use of diagnostic and therapeutic radiation and the avoidance of any unnecessary radiation exposure are currently the most effective preventive strategies that can be recommended.
Biologic Characteristics and Molecular Biology
Salivary Gland Genetic and Molecular Biology
Chromosomal Rearrangements
In recent years, investigations have demonstrated recurrent, nonrandom, and hallmark chromosomal rearrangements, especially translocations that characterize both benign and malignant salivary gland tumors.14 These translocations result in fusion oncogenes that affect the apoptotic threshold, cell cycle regulation, tumor angiogenesis, and growth independence. These gene arrangements have been a more common theme among leukemias, lymphomas, sarcomas, and recently with various epithelial malignancies, including thyroid and breast carcinomas. Characterizing these recurring translocations and their clinical significance is an active area of investigation in hopes of identifying future prognostic and therapeutic targets. Several noteworthy observations have been made to date.
In 2003, the recurrent and hallmark t(11;19)(q21;p13) translocation was described by several groups, demonstrating it to be a key pathogenic event that underlies the development of mucoepidermoid carcinomas.15,16 This translocation has been shown to result in the cyclic adenosine monophosphate–responsive element binding protein (CREB) regulated transcription coactivator 1 (CRTC1)/mastermind-like 2 (Drosophila) (MAML2) fusion transcript that results in a transcription factor acting on the Notch and the CREB pathways.17
Okabe and colleagues have demonstrated that this fusion transcript was associated with a longer disease-free (DFS) and overall survival (OS) on univariate and multivariate analysis.18 Others have reported on its favorable and independent prognostic impact on survival.19,20 Despite this translocation correlating with low-grade mucoepidermoid carcinoma, in multivariate analysis for OS this translocation was found to be independent of tumor grading. Thus this translocation can potentially identify a favorable cohort of high-grade mucoepidermoid carcinomas.21 Lower rates of local and distant relapses have been reported in fusion-positive mucoepidermoid carcinomas.20 The prognostic relevance of this translocation offers not only the promise of future risk-stratified treatment approaches but also that insights into the development of novel therapeutic targets may be gained from further characterization of this fusion gene. For example, unfavorable fusion-positive mucoepidermoid carcinomas are believed to have acquired further somatic mutations conferring increased invasiveness, such as deletion in the CDKN2A (formerly p16) gene.22
Persson and colleagues23 have also demonstrated a recurrent and hallmark t(6 : 9) translocation in adenoid cystic carcinomas resulting in the MYB-NFIB fusion oncoprotein. These investigators suggest that this may also be a key oncogenic event in the pathogenesis of adenoid cystic carcinoma.23
Epidermal Growth Factor Receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (ERBB2)
EGFR and ERBB2 (formerly and often referred to as HER2/neu) are members of the epidermal growth factor receptor transmembrane receptor family and when activated transduce mitogenic signals. Several studies have reported high immunohistochemical expression levels and that it may be more dominantly expressed in non–adenoid cystic carcinomas.24–26 However, the clinical significance of EGFR protein overexpression remains controversial, with multivariate analyses demonstrating both an independent effect27 and no independent association with poor survival.26
Similarly, ERBB2 overexpression has been reported for malignant salivary gland tumors. These include strong overexpression in mucoepidermoid carcinomas28 and in salivary duct carcinomas29,30 but rarely seen for adenoid cystic carcinomas.30 Univariate31,32 and multivariate independent prognostic significance has been reported for ERBB2 overexpression.33,34 Agulnik and colleagues24 suggest that greater clinical activity with the dual EGFR and ERBB2 inhibitor lapatinib may come in identifying tumors with both high EGFR and ERBB2 expression and that ERBB2 may be especially important. Identifying subgroups of patients more dependent on ERBB2 signaling may be important in further defining the therapeutic benefit with ERBB2 inhibition.
c-KIT/CD117
The c-KIT protein is a membrane tyrosine kinase receptor that when activated through binding to the ligands stem cell factor or mast cell growth factor provides signals for cell survival, proliferation, and differentiation. Its overexpression has been particularly noted in adenoid cystic carcinomas35 and has been suggested as one way in which adenoid cystic carcinomas may be distinguished from polymorphous low-grade adenocarcinomas,36 although contradictory findings have also been reported.37
Despite its overexpression, it is clear that it is the nature of any point mutations in the protein that impacts on its response to inhibition with imatinib, a small molecule tyrosine kinase inhibitor that competitively inhibits the activation of the c-KIT receptor and several other structurally similar receptor tyrosine kinases. Although several investigators have not demonstrated the presence of mutations, recent studies using more sensitive polymerase chain reaction techniques confirm the presence of multiple mutations in the c-KIT gene in adenoid cystic carcinomas.38 Although both gain of function (activating function of the receptor) and loss of function c-KIT mutations have been described for other malignancies, the therapeutic implications of these recently described mutations in the management of adenoid cystic carcinomas remain to be determined. Although the independent prognostic significance for c-KIT overexpression has not been evaluated, it has remained an attractive therapeutic target owing to the clinical success of imatinib.
Salivary Gland Tumor Microenvironment
Neoneurogenesis
The study of perineural invasion is an important biologic consideration for many cancers, especially for malignant salivary gland tumors such as adenoid cystic carcinomas. The molecular determinants of this mechanism of spread have only begun to receive attention in recent years. Observations in other cancer sites currently suggest that perineural invasion is the result of a mutual neurotropic interaction with cancer cells characterized by the release of various paracrine growth factors.39 As with neoangiogenesis, it is clear that the ingrowth of nerve endings into a tumor may be stimulated by the tumor release of various neurotrophins such as brain-derived neurotrophic factor (BDNF),40 nerve growth factor (NGF), and its receptor tyrosine kinase A (TrkA).41 Neurotropin staining has been demonstrated to be present in adenoid cystic carcinomas. NGF and TrkA both correlated with the presence of perineural invasion.41 These tumor-innervating nerve cells may release neurotransmitters that function as proliferative and promigratory signals for the tumor cells. Furthermore, nerve fibers are used as routes for tumor cell dissemination. For pancreatic carcinoma, detailed studies of serial histologic sections have demonstrated that tumor cells can progress along branching nerve fascicles in a continuous fashion.42
Hypoxia
Preliminary investigations using methodologies such as immunohistochemical staining for the oxygen-dependent binding of 2-nitroimidazoles suggest that salivary gland malignancies may be well-oxygenated tumors.43 This observation would not support the hypothesis that an underlying mechanism for not only the radioresistance but also the high rates of distant metastasis characterizing malignant salivary gland tumors is the presence of tumor hypoxia. In a study of 12 patients receiving surgery, the hypoxia marker pimonidazole was administered preoperatively. Of the 8 patients yielding sufficient tumor specimen for analysis, immunohistochemical staining was not seen with pimonidazole and for the hypoxia-inducible factor (HIF)-1a and the HIF-1a regulated carbonic anhydrase (CA) IX and glucose transporters (GLUT) 1 and 3. Given that salivary gland malignancies are rare and of diverse histologies, further evaluation is needed before more generalized conclusions can be made.
Pathology and Pathways of Spread
Pathology
The classification of salivary gland tumors is primarily based on morphologic patterns, with two classification systems often recognized. This includes the Armed Forces Institute of Pathology (AFIP) system; and, in 2005, the World Health Organization (WHO), which further developed the classification scheme for salivary cancers originally set forth by Foote and Frazell44 (Table 35-1). The WHO classification includes the following categories: adenomas, carcinomas, nonepithelial tumors, malignant lymphomas, secondary tumors, unclassified tumors, and tumor-like lesions. In contrast, the AFIP classification includes benign epithelial neoplasms, malignant epithelial neoplasms, mesenchymal neoplasms, malignant lymphomas, metastatic tumors, and non-neoplastic tumor-like conditions. Speight and Barrett10 have reported a comprehensive summary of salivary gland histopathology. Given the histologic diversity, this chapter will focus on histopathologies that impact on the pattern of spread and its clinical manifestations.
TABLE 35-1 Classification of Malignant Salivary Gland Tumors
NOS, not otherwise specified.
From Barnes L, Eveson J, Reichart PA, Sidransky D, editors: World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Head and Neck. Lyon, 2005, IARC Press.
Important prognostic factors for disease relapse and survival are summarized in Table 35-2. Practically, histologies may be categorized as high-grade at a greater risk of nodal and distant metastases or low-grade with a lower risk of nodal metastasis. The former includes high-grade mucoepidermoid carcinomas, high-grade adenocarcinoma NOS, carcinoma ex-pleomorphic adenoma, salivary duct carcinomas, and squamous cell carcinomas. Low-grade histologies include low-grade mucoepidermoid carcinomas, adenoid cystic carcinomas, acinic cell carcinomas, and polymorphous low-grade adenocarcinomas. An intermediate group has also been described consisting of intermediate mucoepidermoid carcinomas.
TABLE 35-2 Summary of Prognostic Factors
| Endpoint | Prognostic Factors |
|---|---|
| Local relapse | T3 and T4 category, unresectability/skull base invasion |
| Neck metastasis | Male sex, T3 and T4 category, primary site involving the pharynx, high-grade histology, especially mucoepidermoid and adenocarcinoma NOS |
| Distant metastasis | Clinical perineural invasion, skull base invasion, nodal metastasis |
| Survival | Stage IV, nodal metastasis |
NOS, not otherwise specified.
For mucoepidermoid carcinomas, the histologic grading has been shown to be of prognostic significance. Several three-tiered grading schemes have been shown to be reproducible and predictive of the patient’s outcome by defining low-, intermediate-, and high-grade tumors using five histopathologic features yielding a numerical score for differentiation.45–47 High-grade mucoepidermoid carcinoma has a greater proportion of epidermoid cells than mucus-producing cells, with a solid tumor appearance often mistaken for squamous cell carcinoma. High-grade disease also increases the risk of nodal metastasis sufficient to warrant elective management (e.g., a neck dissection). Despite this, the risk of nodal metastasis with low and especially intermediate grades can be as high as 24% to 30% in some series. High-grade mucoepidermoid carcinomas demonstrated nodal metastases in 56% of patients. In the modified Healey grading schema, lymph node involvement was 0% (low grade), 22% (intermediate grade), and 72% (high grade). T category also is associated with an increased risk of nodal metastasis for both major and minor salivary gland mucoepidermoid carcinomas.48 T1 high-grade disease may be at low risk for nodal metastasis in major salivary glands, that is, anatomic sites not involving the mucosa.48
Pathways of Spread
When considering all histologies together, the risk of lymph node metastasis is increased with T3 and T4 disease, involvement of a pharyngeal site, and high-grade mucoepidermoid carcinomas and adenocarcinoma NOS.49 Table 35-3 shows similar observations with T category, anatomic site, and histology.50 For minor salivary glands, the density of the lymphatics in the anatomic site has a significant influence on the risk of nodal metastases. In general, cancers arising within the oropharynx or nasopharynx have about a 60% incidence of lymph node metastasis compared with 5% to 10% for hard palate and paranasal sinus sites. Minor salivary gland cancers arising within the tongue and floor of mouth have an approximately 40% incidence of lymph node metastasis; those arising within the gingiva have a 20% incidence of lymph node metastasis; tumors of the nasal cavity, buccal mucosa, and lip have a 15% incidence or less.
TABLE 35-3 Incidence of Lymph Node Metastases at Initial Diagnosis by Site, Grade, T Category, Size, and Facial Nerve Paralysis
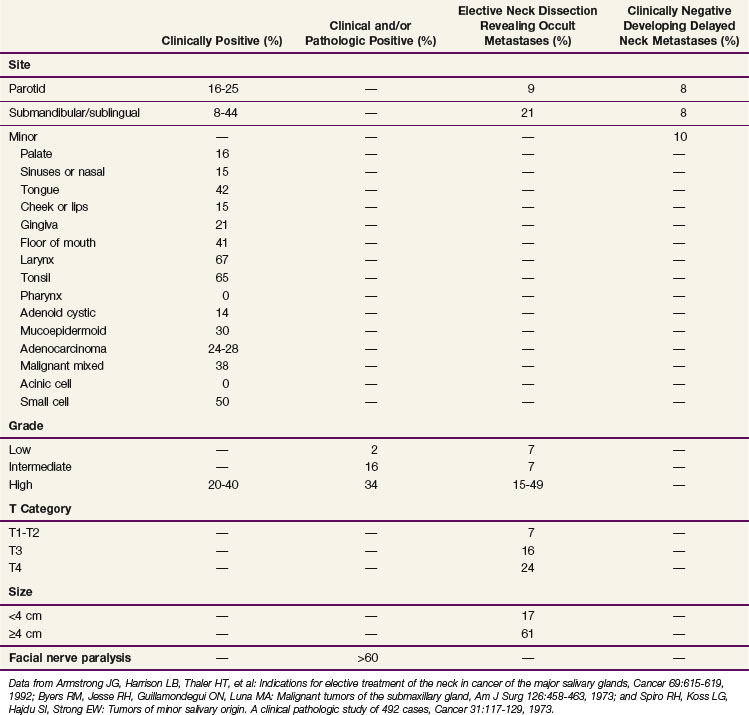
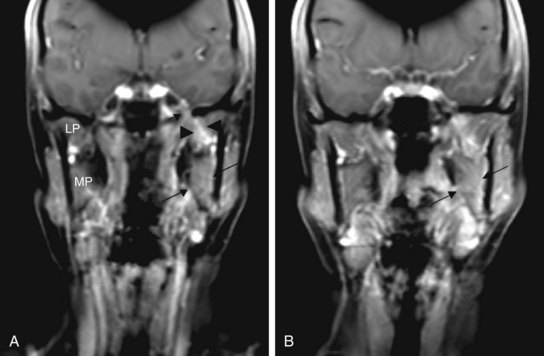
Adenoid cystic carcinomas at the primary site have a predilection for perineural invasion as a unique pattern of local disease extension. Adenoid cystic carcinomas occur most commonly in the palate and frequently spread by perineural invasion. This may be seen in more than 50% of cases. Spread centrally within and peripherally along the nerve are common routes in which this tumor spreads. Growth along the nerve has been shown to have “skip” areas of involvement and noninvolvement, so that a negative nerve margin does not guarantee a final negative margin. However, a recent study of serial sections for pancreatic carcinoma suggests that perineural invasion may, in fact, be contiguous along the branching nerve fascicles and suggest that it may be the branching nature of the nerve and pathologic sampling of the sections evaluated that contribute to the uncertainty in margin assessment.51
The risk of distant metastases is increased with the presence of lymph node metastasis, skull base involvement, and high-grade histology.52 Common distant metastatic sites include lung, liver, and brain and are also associated with the histologic grade.
Clinical Manifestations, Patient Evaluation, and Staging
Clinical Manifestations
Perineural invasion may result in symptoms of pain and numbness but more commonly presents as an asymptomatic mass with an indolent history of growth. Clinical symptoms are more likely to be associated with radiologic evidence of perineural invasion (Fig. 35-1). Extension through the skull base with intracranial growth can also occur and result in mass effect symptoms. Because adenoid cystic carcinomas commonly occur in the palate, spread along the palatine nerves through the greater and lesser palatine foramen is an important route of local spread.
Patient Evaluation
As for any patient with a suspected or documented malignancy, proper evaluation begins with a detailed history and physical examination (Table 35-4). Presence of a new mass in the face, neck, or mouth should be investigated for associated symptoms based on the surrounding anatomic structures that may be affected, thus helping to localize the disease extension. This should also include symptoms that may relate to perineural spread of the cancer. Physical examination should include a full oral cavity inspection and bimanual palpation of structures that are or may be involved. Minor salivary gland tumors typically appear as a submucosal mass and commonly involve the palate. The proximity of the mass and any changes to the greater and lesser palatine foramen should be noted. Flexible fiberoptic nasopharyngolaryngoscopy should be performed to assess for the location and extent of any mucosal lesions in the pharynx or to assess the function of critical cranial nerves that may be suspected to be involved because of a mass at the skull base.
TABLE 35-4 Diagnostic Algorithm for Malignant Salivary Gland Tumors
| General |
| Radiographic Studies |
| Laboratory Studies |
Fine-needle aspiration or ultrasound-guided core needle biopsy of suspicious lesions may be useful to distinguish malignant from benign processes. In one retrospective study of 879 patients, fine-needle aspiration demonstrated a sensitivity of 83% and specificity of 99%, with overall accuracy of 93%.53 However, other studies have demonstrated false-negative rates as high as 33% and 43% for adenoid cystic carcinoma and low-grade mucoepidermoid carcinoma, respectively.54
Both CT and MRI are necessary to assess the extent of disease. Bony destruction and regional lymph node metastases are better visualized with CT, whereas MRI provides information regarding soft tissue infiltration, intracranial spread, and perineural invasion.55 Although the role of PET/CT for salivary gland cancers has yet to be established, early studies have demonstrated an accuracy of more than 90% for detection of the primary tumor and an increased ability in identifying unrecognized local, nodal, and distant metastases.56,57
Staging of Major and Minor Salivary Gland Malignancies
Unlike minor salivary gland tumors, which are staged according to the anatomic site of origin, major salivary gland cancers have their own staging system. In general, tumors of the major salivary glands are staged according to the tumor size and whether there is any extraparenchymal extension (Table 35-5). Extraparenchymal extension is described as either clinical or macroscopic evidence of invasion of the soft tissues. Microscopic extension does not constitute extraparenchymal extension. For parotid tumors the prognostic significance of seventh cranial nerve involvement is also considered. Although tumor grade does affect outcome, the current (7th edition) American Joint Committee on Cancer staging system does not incorporate tumor grade as a component.
TABLE 35-5 Primary Tumor Staging Criteria
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor 2 cm or less in greatest dimension without extraparenchymal extension* |
| T2 | Tumor ≥2 cm but <4 cm in greatest dimension without extraparenchymal extension* |
| T3 | Tumor ≥4 cm and/or tumor having extraparenchymal extension* |
| T4a | Tumor invades skin, mandible, ear canal, and/or facial nerve |
| T4b | Tumor invades base of skull and/or pterygoid plates and/or encases the carotid artery |
* Extraparenchymal extension is defined as the presence of any clinical or macroscopic evidence of invasion of the adjacent soft tissues. Microscopic evidence alone does not constitute extraparenchymal extension for classification purposes.
Nodal category and group staging for major salivary gland tumors follows other head and neck cancer sites and is not described here.58
Primary Therapy
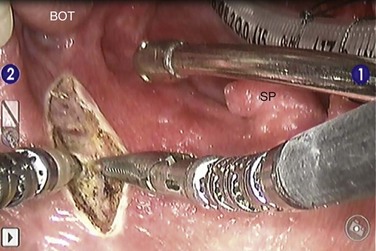
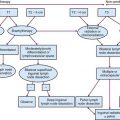
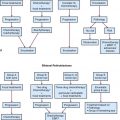
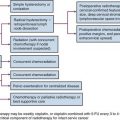
The treatment approach for salivary gland tumors continues to be based on a surgical resectability paradigm. The therapeutic dilemma occurs when there is significant involvement of a functional region in the head and neck involved in speech or swallowing or where unresectable disease occurs. Therapeutic advancements have largely been limited in recent years to the development of novel organ-preserving surgical approaches (Figs. 35-2 and 35-3). Radiotherapy advancements include the development of more conformal radiotherapy techniques and the advent of particle beam therapy. Advancements in systemic agents have included the translation of molecular therapeutics that offer a potentially more favorable therapeutic ratio.
Role of Surgery
The surgical treatment of salivary malignancies can be divided into categories based on the gland involved. The most common site for salivary malignancy is the parotid gland. The submandibular gland and the minor salivary glands have a lower absolute number of cancers; however, the risk of a neoplasm being malignant is higher in submandibular and minor salivary glands than it is in the parotid gland.59
Parotid Gland Malignancy
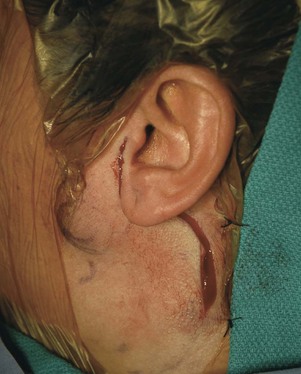
Surgery involves an incision in the preauricular crease extending postauricularly and then into the upper neck (see web-only Fig. 35-1 on the Expert Consult website![]() ). This incision, known as the modified Blair incision, gives access to the seventh cranial nerve as it comes out of the stylomastoid foramen. One of the keys to the procedure is to first identify the nerve, trace it out along its main branches, and then manage the tumor once its relationship to the nerve has been clarified. In cases in which the nerve is compromised by the tumor, or in which the tumor abuts the stylomastoid foramen, it may be necessary to identify the nerve before it comes out of the mastoid bone. This can be accomplished by drilling out the mastoid bone and identifying the nerve before it comes out of the foramen.
). This incision, known as the modified Blair incision, gives access to the seventh cranial nerve as it comes out of the stylomastoid foramen. One of the keys to the procedure is to first identify the nerve, trace it out along its main branches, and then manage the tumor once its relationship to the nerve has been clarified. In cases in which the nerve is compromised by the tumor, or in which the tumor abuts the stylomastoid foramen, it may be necessary to identify the nerve before it comes out of the mastoid bone. This can be accomplished by drilling out the mastoid bone and identifying the nerve before it comes out of the foramen.
Once the appropriate branches of the nerve have been identified, the tumor is removed. Great care is taken to avoid entry into the tumor, and attempts are made to avoid residual positive margins, because this is a known poor prognostic indicator.60,61 Because most tumors involve only the superficial lobe of the parotid, dissecting the tumor carefully off the seventh cranial nerve is appropriate surgical care. Tumors that involve the deep lobe of the parotid gland or that involve the parapharyngeal space just medial to the parotid gland may require extended procedures to perform complete resections. These procedures are often possible through the same parotid incision but occasionally require extended neck incisions or mandible-splitting transoral procedures. Excision of soft tissue, skin, muscle, and neurovascular structures in the region is appropriate if they are involved with tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree