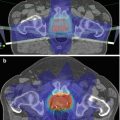
Fig. 6.1
After ligand binding to PSMA the ligand-PSMA complex is internalized, resulting in an effective accumulation of the bound molecule in tumor cells
6.1 Diagnostic Application
Based on the development of small molecule inhibitors, mimicking the endogeneous substrate N-acetyl-l-aspartyl-l-glutamate (NAAG), normally cleaved by N-acetylated alpha-linked acidic dipeptidase NAALADase or glutamate carboxy-peptidase II, several groups engaged in radiolabelled inhibitors with 123I, 99mTc, 18F, 111In, and 68Ga [8–23].
The first high-affinity small-molecule inhibitors of PSMA applied in humans were 123I-MIP-1072 and 123I-MIP-1095. In men with metastatic prostate cancer, SPECT/CT after administration of these molecules demonstrated rapid detection (1–4 h p.i.) of tumor lesions in soft tissue, bone, and the prostate gland [13].
Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)] (68Ga-PSMA-11) became one of the most successful radiopharmaceuticals with respect to on-site availability [12] and clinical application. Figs. 6.2 and 6.3 show patients with a local recurrence and bone metastases, respectively.
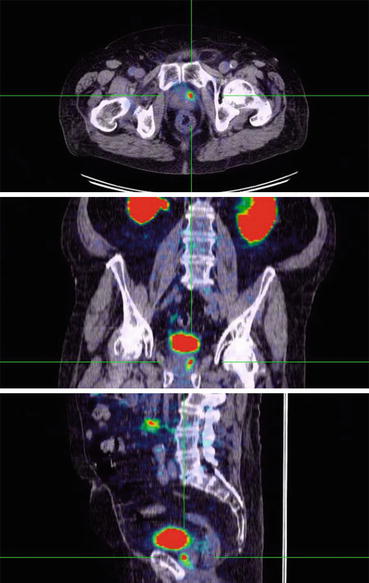
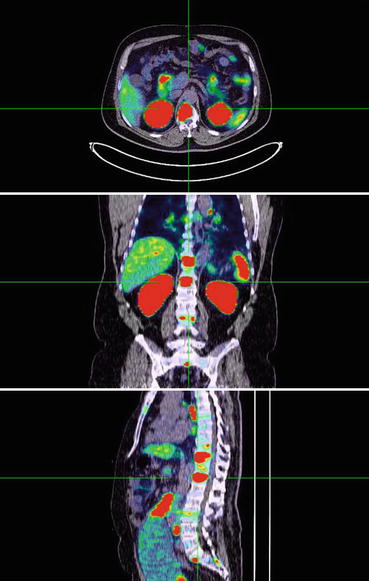

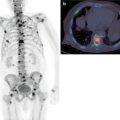
Fig. 6.2
68Ga-PSMA-11 PET/CT scan showing focal uptake in local recurrence of prostate cancer

Fig. 6.3
68Ga-PSMA-11 PET/CT scan showing multiple tracer-avid bone metastases
Two retrospective studies with larger patient numbers (319 and 248 patients) reported detection rates of 82.8 and 89.5% [15, 24]. Tumor detection was positively associated with PSA level and androgen deprivation therapy (ADT). Gleason score (GSC) and PSA doubling time (PSA-DT) were not associated with tumor detection [15, 24]. Furthermore, the detection rates increased with a higher PSA velocity (81.8%, 82.4%, 92.1%, and 100% in <1, 1 to <2, 2 to <5, and ≥5 ng/mL, respectively) [24]. For lesions investigated by histology, 30 were false-negative in four different patients, and all other lesions (n = 416) were true-positive or true-negative. A lesion-based analysis of sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) revealed values of 76.6%, 100%, 91.4%, and 100%, respectively. A patient-based analysis revealed a sensitivity of 88.1% of 116 patients available for follow-up, 50 received local therapy after 68Ga-PSMA-ligand PET/CT [15].
In another retrospective study in 59 patients, the results of the 68Ga-PSMA ligand PET/CT was shown to have a dramatic impact on radiotherapy application with a change of treatment in 52.4% of the cases [25].
Since choline-based PET/CT is widely established for the diagnosis of prostate cancer, a comparison of 18F-fluoromethylcholine- and 68Ga-PSMA-ligand PET/CT has been done in 37 patients with biochemical relapse of prostate cancer showing 78 PCa-suspicious lesions in 32/37 patients using 68Ga-PSMA-ligand PET/CT, wheras 56 lesions were detected in 26/37 patients using choline-PET/CT. The higher detection rate in 68Ga-PSMA-ligand PET/CT concerning PC-suspicious lesions was significant (p = 0.04). All lesions detected by 18F-fluoromethylcholine-PET/CT were also seen by 68Ga-PSMA-ligand PET/CT. In 68Ga-PSMA-ligand PET/CT, SUVmax was clearly (>10%) higher in 62 of 78 lesions (79.1%), and tumor-to-background ratio was clearly (>10%) higher in 74 of 78 lesions (94.9%) when compared to 18F-fluoromethylcholine-PET/CT. Therefore, 68Ga-PSMA-PET/CT detects PC-suspicious relapses and metastases with improved contrast when compared to standard 18F-fluoromethylcholine-PET/CT, especially at low PSA levels [14].
These findings were confirmed by a prospective study in 38 patients [26]. At a PSA value below 0.5 ng/mL, the detection rate was 50% for 68Ga-PSMA versus 12.5% for 18F-fluoromethylcholine. For a PSA between 0.5 and 2.0 ng/mL, the detection rate was 69% for 68Ga-PSMA versus 31% for 18F-fluoromethylcholine. With a PSA higher than 2.0, the detection rate was 86% for 68Ga-PSMA versus 57% for 18F-fluoromethylcholine. In 24/38 (63%) patients, PET/CT had an impact on management, with 54% being due to 68Ga-PSMA imaging alone [26].
Up to now a systematic analysis of the performance of PSMA ligand-based PET/CT is not available for patients with primary tumors prior to standardized surgery and standardized pathological evaluation. However, such an analysis would result in reliable data concerning the sensitivity and specificity of PSMA ligand imaging for tumor and lymph node metastasis detection.
6.2 Endoradiotherapy
PSMA ligands are internalized and accumulate in the late endosomes. Therefore, a therapeutic application of these ligands after coupling to therapeutic isotopes is possible. Since curative approaches no longer exist for patients with metastatic castration-resistant prostate cancer and androgen receptor axis-targeted drugs such as abiraterone and enzalutamide finally lead to resistance against these agents, new isotope-based pharmaceuticals offer the chance of symptom relief and also a possible survival benefit.
Data obtained from the initial clinical investigation of 123I-MIP-1072 and 123I-MIP-1095 led to the evaluation of these radioiodinated ligands as potential PSMA-targeted radiotherapeutics when radiolabelled with 131I [8, 10, 13]. Dosimetry scans with 124I-MIP-1095 PET/CT done in 16 patients showed that the organs receiving the highest absorbed doses following administration of 131I-MIP-1095 are the salivary glands (mean dose 4.6 mGy/MBq), followed by the liver (1.5 mGy/MBq), and the kidneys (1.5 mGy/MBq). This leads to an estimated absorbed dose for the injected therapy activities (mean dose: 4.8 GBq, range 2.0–7.2 GBq) for the salivary glands of 9.2–33.3 Gy. Liver radiation doses fall in the range of 2.9–10.6 Gy. The kidneys received a total absorbed dose between 2.9 and 10.4 Gy. The mean total whole-body absorbed dose was 0.38 mGy/MBq resulting in 0.76–2.7 Gy based on the injected activities. Lymph node and bone metastases were exposed to estimated absorbed doses up to 300 Gy [27].
This was followed by therapy in 25 men with metastatic castration-resistant prostate cancer and PSMA-avid lesions on imaging. The patients received a single therapeutic activity of 131I-MIP-1095 (mean activity: 4.8 GBq, range 2.0–7.2 GBq). Hematological toxicities were mild. The onset of the myelosuppression occurred within 6 weeks post treatment with a quite variable time to recovery, in some cases requiring up to 3–6 months for recovery. White blood cells typically recovered within several weeks, while platelets required several months to recover. Twenty five percent of the patients had a transient slight to moderate dry mouth. No adverse effects on renal function were observed.
In patients with symptomatic bone metastases, 3/13 (23.1%) reported complete resolution of bone pain and 8 (61.5%) a decrease in pain severity. In the remaining 2 patients, the outcome is unknown. In 60.7% of patients, a decline in serum PSA levels of ≥50% was seen [27]. One patient showed a long lasting complete response by serum PSA value and by radiographic imaging. However, in 4/25 patients, an increase of PSA occurred. In responders the median time to PSA progression was 126 days (range 62–469 days). A decrease in PSA was associated with a decrease in number and/or intensity of the lesions visualized on the post-therapeutic PET/CT scan with 68Ga-labeled Glu-NH-CO-NH-Lys(Ahx)-HBED-CC.
While the results obtained with 131I-MIP-1095 show PSMA inhibitors may be effective for radio therapeutic applications, the use of β-particle emitting radionuclides such as 177Lu or 90Y would be preferable, given the advantages of energy, availability, and the potential for on-site labeling via kit formulations. Therefore, PSMA inhibitors have been developed which include chelators for the labeling with radiometals and have similar affinities as the compounds used for diagnostic purposes with excellent tumor uptake and retention. The versatility of DOTA allows the use of beta-emitters, such as 177Lu and 90Y, and alpha-emitters, such as 225Ac, with minimal gamma emissions that can be readily and safely employed in the clinic [22, 28].
Key Points
Prostate-specific membrane antigen (PSMA) is a promising target. PSMA is a type II transmembrane protein with glutamate-carboxypeptidase and folate hydrolase activity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree