Antidepressants
Selective serotonin reuptake inhibitors (SSRI)
Serotonin and norepinephrine reuptake inhibitors (venlafaxine, duloxetine)
Noradrenergic and specific serotonergic antidepressants (mirtazapine, mianserine)
Tricyclic antidepressants
Antipsychotics
Typical antipsychotics (First generation)a
Low-potency
High-potency
Atypical antipsychotics (Second and third generation)
Importantly, studies have shown that psychotropic drugs per se may modify the risk of arterial and venous thromboembolism, which should be taken into consideration when using these drugs in clinical practice. The association with cardiovascular disease is, however, multifactorial and complex. Because psychotropic drugs are widely prescribed, also among patients with cardiovascular disease, understanding the association with thromboembolism and the underlying pathophysiological mechanisms is of great importance and will be reviewed in this chapter.
2 Antidepressants and Arterial Thromboembolism
Ischemic heart disease and stroke are major causes of death worldwide (World Health Organisation 2015). Several studies have addressed the association between antidepressants, particular SSRI, and the risk of arterial cardiovascular events, such as stroke and myocardial infarction, in the general population (MacDonald et al. 1996a; Meier et al. 2001; Bak et al. 2002; Hamer et al. 2011), the elderly (Trifiro et al. 2010), patients with cardiovascular disease (Monster et al. 2004), and in postmenopausal women (Smoller et al. 2009). Noteworthy, the results of studies investigating the risk of myocardial infarction among SSRI users have been inconclusive with some studies suggesting a protective effect (relative risk estimates between 0.4 and 0.9) (Meier et al. 2001; Monster et al. 2004; Smoller et al. 2009; Schlienger et al. 2004; Sauer et al. 2001, 2003; Cohen et al. 2000; Kimmel et al. 2011), others no effect (de Abajo 2011) and even an increased risk (relative risk estimates between 1.4 and 1.9) (Hamer et al. 2011; Tata et al. 2005; Hippisley-Cox et al. 2001; MacDonald et al. 1996b; Blanchette et al. 2008) has been reported.
Recently, a meta-analysis of 13 studies assessed the association between SSRI and stroke found an increased risk of all types of stroke (odds ratio 1.40, 95 % CI: 1.09–1.80), ischemic stroke (odds ratio 1.48, 95 % CI: 1.08–2.02) and hemorrhagic stroke (odds ratio 1.32, 95 % CI: 1.02–1.71) (Shin et al. 2014). This association was more pronounced in the elderly than in the general population group. In the same study, when restricting analyses to studies in which confounding by depression was addressed, the risk of stroke among SSRI users compared with non-users was still significantly elevated. This slightly increased risk may be partly explained by subclinical cardiovascular disease preceding the depression, since depression in elderly people are often caused by “silent infarcts” as a result of an ongoing and long-lasting atherosclerotic process (Taylor et al. 2013; Xekardaki et al. 2012; Wu et al. 2014). Consequently, the use of SSRI might be more frequent among individuals with subclinical cardiovascular disease. Clinical randomized trials are currently exploring potential vascular and neuro protective effects of SSRI treatment in patients with recent stroke, since depression is very frequent in this population and has major impact on mortality and potential rehabilitation (Shi et al. 2014).
With the aim of reducing the risk of recurrent ischemic events, studies have investigated the benefit of antidepressants, especially SSRI. In this context, results have also been equivocal and in most cases based on secondary and post hoc analyses. A meta-analysis of 39 randomized trials from 1967 to 2005 found a tendency towards a lower rate of serious cardiovascular events among SSRI users compared with placebo (odds ratio 0.7, 95 % CI: 0.4–1.2) and a lower rate of non-serious cardiovascular events compared with patients receiving tricyclic antidepressants (odds ratio 0.5, 95 % CI: 0.2–0.9) (Swenson et al. 2006). More recently, a meta-analysis of 5 randomized trials comparing SSRI and placebo among patients with acute coronary syndrome reported no difference in the risk of recurrent myocardial infarction (Mazza et al. 2010).
Possible explanations of the contradictory results may include heterogeneous study populations, different exposure classification, small studies not powered to draw firm conclusions, selection bias, different information sources, and lack of adjustment for cardiac risk factors and socioeconomical status. Finally, confounding by indication is a major problem in some of these studies, as strong evidence indicates that depression is associated with cardiovascular disease, such as coronary artery disease and stroke (Elderon and Whooley 2013; Dong et al. 2012; Kales et al. 2005; Rugulies 2002; Meijer et al. 2011).
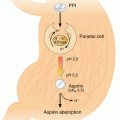
On one hand, SSRI use may be associated with an increased risk of atherothrombotic events, but on the other hand studies have raised concern about an increased risk of bleeding. A recent meta-analysis, which included 22 studies thus examined the association between SSRI and risk of upper gastrointestinal bleeding. Comparing SSRI use with non-use, relative risk estimates ranged from 0.90 to 3.6 with an overall relative risk of 1.55 (95 % CI: 1.35–1.78) (Jiang et al. 2015). In a subgroup analysis, concurrent SSRI and nonsteroidal anti-inflammatory drug use and combined use of SSRI and antiplatelet drugs further increased the risk of bleeding. In the same study, the association with gastrointestinal bleeding was strongest for paroxetine (1.68, 95 % CI: 1.08–2.26), sertraline (1.67, 95 % CI: 1.37–2.04), fluoxetine (1.77, 95 % CI: 1.32–2.38), citalopram (2.07, 95 % CI: 1.47–2.92), and escitalopram (2.45, 95 % CI: 1.35–4.42), while no significant association was found among patients using fluvoxamine and venlafaxine (a serotonin and norepinephrine reuptake inhibitor).
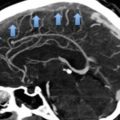
The association between SSRI and intracranial hemorrhage has also been studied. Although a recent meta-analysis found that SSRI use compared with non-use was significantly related to an increased risk of intracranial hemorrhage (Hackam and Mrkobrada 2012) other studies reported no association (de Abajo 2011). In the same meta-analysis, a tendency towards an increased risk of hemorrhagic stroke (defined as intracerebral or subarachnoid hemorrhage) and no association with subarachnoid bleeding was reported in patients treated with SSRI. In patients concomitantly using SSRI and oral anticoagulants, the risk of bleeding has been shown to increase compared with patients using oral anticoagulants only (Hackam and Mrkobrada 2012; Quinn et al. 2014). Studies comparing use of SSRI to non-use on the risk of perioperative bleeding (coronary artery bypass graft surgery, postpartum, orthopedics, and breast cancer) have reported contradictory results (de Abajo 2011).
3 Potential Mechanisms Between Antidepressants and Arterial Atherothrombotic Events
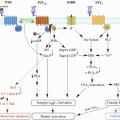
The clinical effects of SSRI are mainly linked to inhibition of the serotonin reuptake transporter (5-HTT) (Berger et al. 2009). Serotonin is synthesized by the enterochromaffin cells in the gut and mainly transported in dense granules by platelets; however, there is limited passage of serotonin across the blood brain barrier. In the central nervous system, serotonin is almost exclusively produced by neurons in the brainstem. The central serotonin regulation is important for appetite, mood, sleep, cognition, temperature and motor control (Jacobs and Azmitia 1992), whereas the cardiovascular roles of serotonin include regulation of heart rate, blood pressure, vascular tone, and platelet aggregation (Berger et al. 2009).
In addition to its effects in neurons, the SSRI receptor has also been found in platelets, smooth muscle cells, and intestinal epithelial cells with the potential to exert extra-neuronal effects (Berger et al. 2009). Since platelets play a key role in cardiovascular disease, the effect of SSRI on platelets has been studied extensively (Maurer-Spurej 2005). In patients treated with SSRI for several weeks, SSRI have been suggested to cause depletion of serotonin storage in platelets (Halperin and Reber 2007), attenuate platelet adhesion to collagen and fibrinogen (Halperin and Reber 2007), and to potentiate the aggregation induced by adenosine diphosphate, epinephrine and collagen (Halperin and Reber 2007). Theoretically, this may lead to bleeding – or to cardiovascular protection in patients at increased risk of thrombosis. Serotonin also plays a role as a potent vasoactive substance, which may cause vasoconstriction in cerebral arteries leading to ischemic stroke (Muhonen et al. 1997). Apart from its inhibitory effects on platelet function, rat studies have indicated that SSRI (paroxetine) might have a direct toxic effect on gastric mucosa (Takeuchi et al. 2011; Yamaguchi et al. 2008). Consistent with this finding and the ability of SSRI to inhibit platelet aggregation, a higher prevalence of gastric mucosal injuries in patients treated with SSRI compared with control patients has been reported (Dall 2010).
4 Clinical Implications
Due to contradictory findings and sparse data, the link between antidepressants and arterial events remains to be fully established. If any association exists, antidepressants most likely only play a minor role as a risk factor for arterial cardiovascular events compared with classical risk factors for atherothrombosis. On the other hand, use of SSRI may increase the risk of bleeding; however, compared with antiplatelet drugs such as aspirin and clopidogrel, the risk is only moderate. Nonetheless, co-treatment with proton pump inhibitors may decrease the risk of upper gastrointestinal bleeding and should therefore be considered (Jiang et al. 2015; de Abajo and Garcia-Rodriguez 2008). In patients with other risk factors for bleeding (high age, previous bleeding, use of NSAID, antiplatelets or oral anticoagulants) caution is advised when prescribing SSRI. In addition, in order to reduce the risk of bleeding, it is important to make a thorough review of these patients’ medication list and e.g. reduce dose of glucocorticoid or change treatment with NSAIDs to Paracetamol. The potential antiplatelet and neuro protective effects of SSRI are currently being investigated in three large randomized clinical trials on patients with recent stroke (clinicaltrials.gov) (Kraglund et al. 2015).
5 Antipsychotics and Arterial Events
A systematic review published in 2011 studied the association between antipsychotic drugs and myocardial infarction. However, due to substantial clinical and methodological heterogeneity between the five studies in the review, no firm conclusions were possible, albeit the largest included study demonstrated no association (Brauer et al. 2011). In contrast, and more recently, a self-controlled case series study from the United Kingdom (UK) found an increased 30-day risk of myocardial infarction following initiation of antipsychotic treatment with higher risk estimates (non-significant) for typical than atypical antipsychotics (Brauer et al. 2014). This finding was partly supported by a Taiwanese study of 59,806 psychiatric patients later found to have a myocardial infarction. The study showed that use of any type of antipsychotics 30 days prior to myocardial infarction increased the risk significantly (odds ratio 2.52, 95 % CI: 2.37–2.68). The use of amisulpride was associated with the highest risk of myocardial infarction. In contrast to the study from UK, a higher risk of myocardial infarction was found among patients using atypical compared with typical antipsychotics (odds ratio 1.63, 95 % CI: 1.29–2.05) (Lin et al. 2014).
The association between antipsychotics and stroke has received attention since 2002, when four randomized studies in dementia patients with 1–3 months of follow-up found an increased risk of cerebrovascular events in patients receiving risperidone (4 %) compared with placebo (2 %) (Wooltorton 2002). Although some contradictory findings have been reported, antipsychotics increase the risk of stroke in demented patients (Sacchetti et al. 2010a). The risk of stroke associated with typical and atypical antipsychotics seems to be similar (Sacchetti et al. 2010a). A study found that the risk of stroke was 12.4-fold (95 % CI: 8.4–18.1) higher in antipsychotic drug users in the first month of treatment compared with non-users. After this initial period, the risk returned to almost normal levels (Sacchetti et al. 2010b).
6 Potential Mechanisms Between Antipsychotics and Arterial Atherothrombotic Events
The mechanisms underlying the possible association between use of antipsychotics and risk of arterial events have not been established. Hypotheses about drug-induced obesity, hyperleptinaemia, antiphospholipid antibodies, and an increased activity in the coagulation system might add to the increased risk of arterial events (Hagg and Spigset 2002). Antipsychotics have also been associated with an increased risk of diabetes and hence they may also increase the risk of arterial events (Sohn et al. 2015). Again, confounding by indication may play an important role, because patients receiving long-term antipsychotic treatment mainly suffer from schizophrenia and thus tend to have lower socio-economic status, unhealthy lifestyle, increased smoking, alcohol and drug abuse, less somatic hospitalization than needed, and are less likely to receive invasive cardiac procedures compared with matched controls (Laursen and Nordentoft 2011).
7 Clinical Implications
Despite the need for further investigation, awareness of a possible increased risk of myocardial infarction and stroke in patients prescribed with typical or atypical antipsychotic drugs especially in the early period after initiation of treatment is essential. Physicians should hold particular attention towards any new cardiac and neurological symptoms in patients initiating antipsychotic treatment. Especially in elderly patients with dementia, use of antipsychotics should be prescribed with great caution. Importantly, many psychotic patients do not react appropriately to alarm symptoms indicating cardiovascular disease, as many individuals with schizophrenia are less sensitive to pain than normal individuals (Dworkin 1994; Potvin and Marchand 2008).
8 Antidepressants and Venous Thromboembolic Events
Venous thromboembolism (VTE) is a common disorder affecting nearly one in 1000 adults annually (Oger 2000). It is an important and potentially preventable disease accounting for substantial morbidity and mortality (Kobberoe Sogaard et al. 2014). Known precipitants for VTE include acquired risk factors such as pregnancy, surgery, immobilization or active malignancy, and inherited risk factors such as deficiency of protein S or protein C, and factor V Leiden mutation (Goldhaber 2010).
Studies examining the association between antidepressants and VTE are limited and ambiguous. A case-control study conducted in New Zealand found an almost fivefold (odds ratio 4.9, 95 % CI: 1.1–22.5) increased risk of fatal pulmonary embolism in users of any antidepressant drug compared with non-use (Parkin et al. 2003). This finding was supported by another case-control study from the Netherlands reporting a 2.3-fold increased risk for venous thromboembolism in users of antidepressants compared with non-users (odds ratio 2.3, 95 % CI: 0.6–10.2) (Thomassen et al. 2001). Both studies were, however, small and confidence intervals accordingly wide. Furthermore, both studies are >10 years old and neither discriminated dose or type of drug, or accounted for the important fact that the frequency of smoking is markedly higher among patients with depression. A more recent, nested case-control study from UK compared current use of antidepressant drugs with non-use and also addressed the specific type of drug (Jick and Li 2008). In this study, use of tricyclic antidepressants was associated with a small but significantly increased risk of VTE (odds ratio 1.4, 95 % CI: 1.1–1.8), whereas no increased risk was found for SSRI. In a recent Taiwanese study, an approximately 1.5-fold increase in VTE risk was reported for users of any antidepressants (odds ratio 1.59, 95 % CI: 1.27–2.00) (Wu et al. 2013), and as opposed to the UK study, both tricyclic antidepressants and SSRI showed increased risk of VTE (tricyclic antidepressants: odds ratio 1.56, 95 % CI: 1.11–2.18; serotonin 5-HT2A receptor blockers: odds ratio 2.03, 95 % CI: 1.27–3.24; and antidepressants with a low potency of serotonin reuptake inhibition: odds ratio 1.57, 95 % CI: 1.18–2.08).
Contrary to the studies above, several studies have reported no association between antidepressants and VTE. A case-control study of 214 patients aged <60 years thus reported no association between antidepressant use and subsequent risk of VTE when controlling for antipsychotic drug exposure (odds ratio 1.7, 95 % CI: 0.8–3.7) (Zornberg and Jick 2000). This null result was supported by a larger (n = 1354) and more recent case-control study reporting an odds ratio of 1.1 (95 % CI: 0.9–1.5) (Lacut et al. 2007) and by a cohort study of patients aged age > 65 years where users of antidepressants were compared with users of thyroid replacement therapy (hazard ratio 1.02, 95 % CI: 0.91–1.14) (Ray et al. 2002).
9 Clinical Implications
The evidence of increased risk of VTE among users of antidepressants is sparse and inconsistent. The increased risk reported in some studies is modest and most pronounced for tricyclic antidepressants. The association, if any, is weak and the psychiatric indication of treatment is therefore the primary determinant when prescribing antidepressants. However, in patients with a strong predisposition or history of VTE, the psychiatric indication of use should be substantial, and treatment with SSRI should presumably be preferred. Further studies examining the type of drug, dose and duration of use are warranted.
10 Antipsychotics and Venous Thromboembolism
The evidence for an association between antipsychotics and VTE is more robust and consistent than the association between antidepressants and venous thromboembolism. Current evidence is based primarily on case-control and cohort studies.
In 2011, a meta-analysis of seven case-control studies concluded that use of antipsychotics was associated with an almost 2,5-fold increase in the risk of VTE (odds ratio 2.39, 95 % CI: 1.71–3.35) (Zhang et al. 2011). Pooled estimates by drug type showed that use of low-potency antipsychotics (odds ratio 2.91, 95 % CI: 1.80–4.71) is the most important risk factor for VTE followed by atypical (odds ratio 2.20, 95 % CI: 1.22–3.96), typical (odds ratio 1.72, 95 % CI: 1.31–2.24) and high-potency drugs (odds ratio 1.58, 95 % CI: 1.50–1.67). The results of this meta-analysis was driven by two important case-control studies of which a Danish study reported a more than twofold increased risk of VTE compared with non-users (odds ratio 2.27, 95 % CI: 1.55–3.31) (Jonsson et al. 2009) and a study from UK reported an almost two-fold increase (odds ratio 1.80, 95 % CI: 1.49–2.18) (Parker et al. 2010). More recently, a meta-analysis of 12 observational studies (case-control and cohort studies) reported a more moderate increase in the risk of VTE (odds ratio 1.54, 95 % CI: 1.28–1.86) (Barbui et al. 2014). In the same study, an analysis restricted to cohort studies (n = 5) revealed an even smaller risk of VTE (odds ratio 1.34, 95 % CI: 1.13–1.58).
11 Potential Mechanisms Between Antipsychotics and Venous Thromboembolism
The underlying causal mechanisms explaining the association between antipsychotics and VTE have not been clarified yet, although numerous mechanisms have been proposed. Antipsychotic drugs, especially the atypical drugs, are known to cause weight gain and metabolic syndrome, which are well known risk factors for VTE (Osborn et al. 2008). The causal pathway may also include drug-induced sedation or physical restraining, which consequently increases the risk of VTE through immobility and venous stasis. Increased aggregation of platelets has also been suggested for typical antipsychotics (Boullin et al. 1975; Orr and Boullin 1976). It may constitute a confounding problem that the vast majority of studies included in the aforementioned meta-analyses did not adjust for the psychiatric disease per se (Chapelle et al. 2013) or complications of the disease, such as a sedentary lifestyle, poor diet, smoking, and drug abuse (Hagg et al. 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree