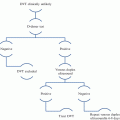
Fig. 1
PRISMA flow diagram of systematic review evaluating the role of neuromuscular stimulation in venous disease
4 Physical Parameters
Comparison between trials is hindered by variations in protocols and outcome measures, however some investigators have made comparisons varying parameters of electrical stimulation. All studies showed an improvement in venous haemodynamics with stimulation of the calf muscle pump compared to rest:
Volume flow in the calf increased 50–719 % from baseline (Heath and Gibbs 1992) (venous occlusion plethysmography), 60–614 % (ultrasound) (Broderick et al. 2009, 2010a, 2011, 2013; Izumi et al. 2010)
Femoral and popliteal peak velocity increased by 25–650 % (Broderick et al. 2009, 2010a, b, 2013; Izumi et al. 2010; Lyons et al. 2002; Kaplan et al. 2002; Tucker et al. 2010) (ultrasound)
Time averaged maximum velocity (TAMV) increased by 178–354 % (Broderick et al. 2013).
Ejection volume due to electrical stimulation was 60–100 % of that elicited by voluntary contraction (Faghri et al. 1998; Miller et al. 2000) and popliteal venous velocity “strongly correlated” to force of plantar flexion (Corley et al. 2009).
Calf vascular resistance was significantly reduced after electrical stimulation (Miller et al. 2000), equivalent to post-exercise (voluntary calf contractions to a metronome).
Kaplan et al. found that stimulation of the foot and calf are equivocal in their effect on popliteal flow (Kaplan et al. 2002).
4.1 Effect of Electrical Parameters on Venous Haemodynamics
4.1.1 Direct Stimulation of the Muscle
Nicolaides et al. report that electrical stimulation of the muscle directly using pulse width 50–100 ms and frequency 25 mHz (15/min) produced maximal venous velocity without discomfort (Nicolaides et al. 1972). Settings above this were untenable. Griffin et al. increased the frequency of stimulation between 2 and 120 stimulations per minute and found that the popliteal vein peak velocity increased for stimulations up to 10 mHz, but decreased towards 2 Hz (Griffin et al. 2010). Ejected calf volume per minute increased from 20 to 120 ml/min with increasing stimulation frequency.
4.1.2 Indirect Stimulation
Lyons et al. found that pulse duration 300 μs and frequency of 35 Hz applied to nerve produced the greatest increase in popliteal peak venous velocity with a pulse width 200–300 μs, and frequency 24–35 Hz (Lyons et al. 2002). Tucker et al. found that increasing stimulation frequency from 1 to 5 Hz and pulse amplitude from 1 to 40 mA had a positive correlation with increasing venous volume flow peak venous velocity and microcirculatory flux (Tucker et al. 2010).
Izumi et al. (2010) compared two different electrical stimulation frequencies (10 Hz vs 50 Hz) using the same stimulator and found that the lower frequency produced a higher peak velocity compared to 50 Hz stimulation, however the findings seem to be confounded by a mix of direct and indirect stimulation.
4.2 Comparison of NMES to Other Medical Devices
Four studies compared the effect of electrical stimulation to IPC (Laverick et al. 1990; Czyrny et al. 2010; Williams et al. 2014b; Jorgensen et al. 1994). Both indirect stimulation via the common peroneal nerve, and direct stimulation of calf muscles have been shown to be non-inferior to calf and/or foot IPC.
On comparing calf IPC to electrical stimulation via the common peroneal nerve, Williams et al. (2014b) demonstrated that whilst peak venous velocity increased significantly with both methods, only NMES had a significant effect on time averaged mean velocity (TAMV) and volume flow. On comparing foot IPC to calf NMES, Laverick et al. demonstrated a greater increase in mean venous velocity and peak venous velocity with NMES.
Faghri et al. demonstrated increased cardiac stroke volume (24 %), cardiac output (26 %) and a reduced total peripheral resistance (21 %) with electrical stimulation when compared to IPC of the calf and thigh (Faghri et al. 1997).
Lyons et al. demonstrated that the effect of electrical stimulation is augmented by a factor of 2 with the addition of graduated compression stockings (Lyons et al. 2002).
Nicolaides reported an increased incidence of peri-laparotomy DVT with NMES when compared to a combination of graduated compression stockings and calf/thigh IPC (18 % vs 4 %) (Nicolaides et al. 1983).
5 Efficacy of Clinical Application
5.1 Electrical Stimulation as a Method of Thromboprophylaxis
Outcome parameters for DVT detection in these studies are heterogenous, and include presence of clinical symptoms, phlebography, I-125 fibrinogen uptake tests and duplex ultrasound.
Between 1967 and 1973, four case series looked at treatment of one leg with direct muscle stimulation, using the contralateral leg as a no-treatment control. An absolute risk reduction (ARR) of 2–12.7 % was seen when compared to control leg (Doran and White 1967; Doran et al. 1970; Browse and Negus 1970; Dejode et al. 1973).
Randomised trials with subjects undergoing open abdominal surgery, used direct muscle stimulation of the calf versus heparin versus no-treatment controls. This showed a DVT ARR 16–26.3 % (Nicolaides et al. 1972; Becker and Schampi 1973; Lindstrom et al. 1982; Pollock 1977) for NMES, and 26–54 % for subcutaneous heparin three times daily. There was a 2.1 % declared major haemorrhage rate requiring transfusion for those on heparin, whereas there were no adverse events reported with NMES (Rosenberg et al. 1975). One study reported that NMES gave a DVT ARR 39 % over no-treatment control when the laparotomy indication was for malignancy (Lindstrom et al. 1982). One paper reports of NMES use in major trauma, where heparin is contraindicated for VTE prophylaxis – DVT incidence was equivocal (27 % NMES versus 29 % control, n = 47) (Velmahos et al. 2005). Studies in surgical patients comparing heparinised patients with or without NMES have mixed results, some showing no difference in DVT rates (Bostrom et al. 1986), whilst other show ARR DVT 22.5 % and ARR death 5 % (Lobastov et al. 2014). When combined together, NMES and heparin gave an ARR 40.4 % when compared to placebo (Merli et al. 1988).
Figures 2, 3 and 4 summate meta-analysis of NMES under three conditions. NMES versus no treatment favours NMES, with DVT odds ratio 0.4 (p < 0.0001). NMES versus heparin results in DVT odds ratio 1.84 (p = 0.03), whilst NMES and heparin versus heparin alone give an odds ratio 0.07 (p < 0.001).
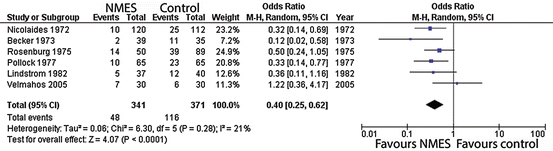
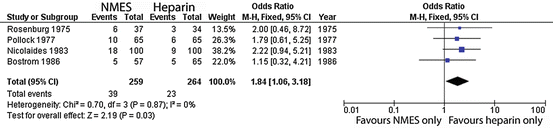


Fig. 2
Meta-analysis of trials evaluating deep venous thrombosis risk: NMES versus no-treatment control

Fig. 3
Meta-analysis of trials evaluating deep venous thrombosis risk: NMES versus heparin

Fig. 4
Meta-analysis of deep venous thrombosis risk: dual therapy with NMES and heparin versus heparin alone
5.2 Effect of Electrical Stimulation on leg Oedema/Chronic Venous Disease
Five studies examined the effect of NMES on oedema, four of which were on healthy individuals (Broderick et al. 2010a; IO et al. 2003; Green et al. 2008; Goddard et al. 2008) and one study involved patients with evening oedema (Bogachev et al. 2011). Healthy volunteers showed significant increase in foot and ankle volume when standing still over 30 min, which was abolished with NMES use (IO et al. 2003). Both leg volume measurements and air plethysmography, in the seated position, demonstrated reversal of fluid pooling in the legs with electrical stimulation of the calf (Green et al. 2008; Goddard et al. 2008). Broderick tested leg swelling in supine subjects, and unsurprisingly showed no leg swelling over 4 h bed rest, and no additional effect with NMES (Broderick et al. 2010a).
In trials on patients with venous disease, 20 min of treatment over a 30 day period resolved evening oedema in 59.4 % of cases, reduced it in 34.4 %, and remained unchanged in 6.2 % of cases (Bogachev et al. 2011). This resulted in a significant reduction in group average supramalleolar circumference, reduced pain score and improved quality of life.
5.3 The Effect of Electrical Stimulation in Patients with Impaired Calf Muscle Pump
Patients with neurological disorders affecting the lower limb will suffer from impaired muscle pump activity. In addition to increasing their risk of developing deep vein thrombosis, this group of patients are at a higher risk of cardiovascular failure because of autonomic dysfunction and have impaired venous return to the heart. Van Beekvelt et al. (2000) using strain gauge plethysmography demonstrated that electrical stimulation can improve muscle pump activity in spinal cord injured patients. Although the spinal cord injury patients could tolerate higher current (60 mA), their muscle pump action was significantly lower than that of able bodied subjects (21.5 % vs 67.7 %). Effects of long term treatment were not investigated.
6 Discussion
Electrical stimulation has been shown to improve venous haemodynamics, however the reporting of stimulation parameters varies greatly between trials. It is not clear from the evidence which are the optimum parameters for stimulation, and it may be that clinical indication will dictate whether direct or indirect stimulation is most suitable. Electrode placement determines the effectiveness of calf muscle stimulation due to the particular nerve or muscle bulk targeted, with direct comparisons being misleading. The contraction relaxation times are variable which would affect venous filling and therefore the calf muscle pump function. Subject position is heterogenous, and comparing studies with standing, seated, prone and supine subjects may be injudicious. Haemodynamic outcome measures include air plethysmography, photoplethysmography, strain gauge plethysmography, venous occlusion plethysmography and venous duplex. The site of duplex scanning varies between the femoral, popliteal, posterior tibial and peroneal veins, whilst assorted haemodynamic parameters are reported, and the clinical significance of each parameter is poorly understood. Similarly, the techniques for detecting DVT in the initial studies lacked the sensitivity and specificity of today’s imaging techniques.
NMES is non-inferior to IPC in terms of venous haemodynamics, and does not carry the complications associated with IPC (mainly excessive heat and sweating under the inflatable cuffs). However some subjects have found NMES uncomfortable (Lachmann et al. 1992). One of the benefits of electrical stimulation over IPC is that the action increases the activity of the users own muscles, as opposed to a passive compression system. A randomised control trial of intensive care patients demonstrated an improvement in muscle strength with electrical stimulation (Karatzanos et al. 2012). It has been shown that aerobic exercise of any type, even just the arms, has a positive effect on walking distances in claudicants, and may alter metabolic profile (Zwierska et al. 2005). The cardiovascular benefits from NMES are unknown.
Venous stasis is thought to be a major contributor in the pathogenesis of deep vein thrombosis, and is the main mode of action targeted by electrical stimulation in VTE prevention (Mackman 2012). In our meta-analysis, heparin has been shown to be most efficacious in the prevention of DVT when compared to NMES alone. However where heparin is contraindicated, NMES significantly reduces VTE risk. In cases of very high risk, the addition of NMES to a heparin thromboprophylaxis regime increases efficacy. Katz observed a fibrinolytic effect with the use of NMES, and when twinned with increased venous velocities in the deep veins may work in synergy with heparin (Katz et al. 1987). It must also be kept in mind that there are significant risks associated with heparin, such as major haemorrhage, stroke, exacerbation of post-operative bleeding, heparin-induced thrombocytopaenia, and osteoporosis. There is also a need to adjust dose in extremes of body mass, pregnancy and renal impairment (Gouin-Thibault et al. 2005). These problems are not encountered with NMES. High risk peri-operative patients are likely to benefit from electrical stimulation in addition to low molecular weight heparin. In particular, patients who are regarded as high risk such as those undergoing bariatric surgery and laparoscopic cancer surgery, or immobile patients with spinal cord injuries. The combination of NMES with graduated compression stockings may further enhance the haemodynamic effects.
The potential clinical application of electrical stimulation in venous disease is largely unexplored, but given the dependence of the venous system on the calf muscle pump, and the large numbers of people with CVD, this is worth exploring. Given that orthostatic oedema can be reversed with NMES, and the ability of the calf muscle pump to be trained over time, NMES may be very successful in this area. Increased impedance of skin and subcutaneous tissues in the presence of oedema requires higher stimulation settings to achieve muscle contraction, but may be limited by pain threshold. Venous haemodynamic measurements tell part of the story, but we recommend future trials should concentrate on translating these effects into clinical practice. Clinical outcomes (e.g. ulcer healing rates) and quality of life data will be most useful in analyzing NMES as a clinical tool, especially given the success of IPC in this cohort (Dillon 1986; Kolari and Pekanmaki 1986; Kolari et al. 1988; Smith et al. 1990; Nelson et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree