Chapter 69 Respiratory Disease
Patients with human immunodeficiency virus (HIV) infection can develop respiratory dysfunction due to a wide variety of disorders. Although the immediate focus is often directed at opportunistic infection (OI), clinicians caring for HIV-infected patients presenting with dyspnea, cough, chest pain, with or without fever, must approach the differential diagnosis methodically. In addition to HIV-associated OIs, patients with HIV infection are also susceptible to community-acquired pathogens (e.g., influenza or Mycoplasma) and hospital-acquired organisms (e.g., methicillin-resistant Staphylococcus aureus or extended-spectrum β-Lactamase-producing enteric organisms). Risk factors for HIV infection, such as injection drug use, may contribute to pulmonary disease (e.g., endocarditis with septic pulmonary emboli, aspiration pneumoniasecondary to altered mentation, noncardiogenic pulmonary edema, talc granulomatosis). Clinicians should also recognize that HIV-infected persons might have preexisting or concurrent chronic pulmonary disease (e.g., asthma or chronic obstructive pulmonary disease) unrelated to their HIV infection. Thus, a rational approach to respiratory dysfunction in patients with HIV infection should include the same considerations as any immunologically normal patient, as well as consideration for the processes that occur with special frequency or severity in patients with HIV infection (Table 69-1). This chapter presents an approach to lower respiratory tract and upper respiratory tract infections in this patient population.
Table 69-1 Causes of Pulmonary Dysfunction Seen with Increased Frequency or Severity in Patients with HIV Infection
| Opportunistic Infections |
VZV, varicella-zoster virus.
a Cytomegalovirus (CMV) and Mycobacterium avium complex (MAC) are rarely the cause of pulmonary dysfunction in patients with HIV infection regardless of their CD4+ T-lymphocyte and neutrophil counts.
b Although disseminated Strongyloides is an AIDS-defining disease, it has rarely been documented in patients with HIV infection.
EPIDEMIOLOGIC PERSPECTIVE
The clinical setting–outpatient, inpatient, or ICU–influences the relative frequency of respiratory diseases that are observed. The Pulmonary Complications of HIV Infection Study (PCHIS) was a prospective, observational cohort study that followed more than 1 150 HIV-infected subjects for ∼5 years at six sites across the United States prior to the era of highly active antiretroviral therapy (HAART).1,2 PCHIS documented that patients who presented to an outpatient clinic with respiratory illness more often had upper respiratory tract infections and acute bronchitis than pneumonias due to bacteria or Pneumocystis.2 In contrast, bacterial pneumonia and Pneumocystis pneumonia (PCP) are the most frequent causes of pneumonia in hospital-based series. Pneumonias due to bacteria (Streptococcus pneumoniae: 21% of all pneumonias in HIV-infected subjects) and Pneumocystis (27%) greatly exceeded those due to viruses, mycobacteria, and fungi (each <5%) in a year-long hospital-based study of community-acquired pneumonia at Johns Hopkins Hospital.3 PCP (35%) and bacterial pneumonia (27%) were also the most common etiologies reported in a hospital-based study of community-acquired pneumonia from Atlanta.4 Finally, PCHIS showed that the most common pulmonary diseases observed among HIV-infected patients requiring critical care were PCP followed by bacterial pneumonia.5 These results from the multicenter PCHIS are similar to those reported from single institutions such as San Francisco General Hospital6–8 as well as cohorts in Europe.9
The geographic location of the clinic or hospital may also influence the frequency of the diagnoses. In specific populations or geographic regions, mycobacterial and endemic fungal pneumonias are important considerations. Worldwide, tuberculosis (TB) is a leading cause of death. In endemic areas, diseases caused by Histoplasma capsulatum or Coccidioides immitis are among the most frequent infections seen,10 whereas at San Francisco General Hospital they are rarely encountered. Table 69-2 lists diagnostic clues to the causes of respiratory disease in patients with HIV infection.
Table 69-2 Approach to Respiratory Disease: Diagnostic Clues
COPD, chronic obstructive pulmonary disease; IDU, injection drug users; MSM, men who have sex with men; PCP, Pneumocystis pneumonia; PPD, purified protein derivative.
In HIV-infected persons, PPD is considered positive if ≥5 mm induration.
a Most frequent causes of respiratory illness among HIV-infected outpatients from the multicenter Pulmonary Complications of HIV Infection Study.
b Most frequent causes of pneumonia among HIV-infected persons hospitalized at San Francisco General Hospital 1996–98 (Huang, unpublished data). PCP and Streptococcus pneumoniae were the two most common causes of community-acquired pneumonia among HIV-infected persons hospitalized at Johns Hopkins Hospital, 11/90 to 11/91.
c Most frequent causes of pneumonia among HIV-infected persons admitted to intensive care from the multicenter Pulmonary Complications of HIV Infection Study.5
LOWER RESPIRATORY TRACT DISEASE: PNEUMONIA
One of the first decisions that clinicians face is whether or not to place a patient in isolation. Suspicion of TB, influenza, or respiratory syncytial virus (RSV) infection based on history, clinical presentation, chest radiograph, or initial laboratory tests are examples of situations that would mandate respiratory (e.g., TB or influenza) or contact (e.g., RSV) isolation. Clinicians must recognize the risk that transmissible respiratory pathogens pose to other patients, hospital staff, and visitors. It is far preferable to be liberal in the use of isolation precautions until the specific diagnosis is established than to risk the spread of transmissible pathogens. Respiratory isolation is especially important to consider during procedures likely to increase pathogen aerosolization such as sputum induction, bronchoscopy, or intubation.
HIV TRANSMISSION CATEGORY AND HABITS
A patient’s HIV transmission category and habits influence the relative frequency of various HIV and nonHIV-associated pulmonary diseases (Table 69-2). Kaposi sarcoma is seen almost exclusively in men who report having sexual contact with other men (MSM). Approximately 95% of the cases of HIV-associated Kaposi sarcoma occur in MSM; and although described, the occurrence of Kaposi sarcoma in women is quite uncommon.11 Bacterial pneumonia and TB are more common in HIV-infected patients who are injection drug users than in HIV-infected patients without a history of injection drug use.12,13 The PCHIS found that the rate of bacterial pneumonia was 11.1 episodes per 100 person-years among injection drug users, compared with 4.1 and 3.8 episodes per 100 person-years among MSM (P < 0.001) and female heterosexuals (P = 0.003), respectively.12 Furthermore, injection or other illicit drug use can cause a variety of acute nonHIV-related pulmonary diseases (e.g., aspiration pneumonia secondary to central nervous system depression, endocarditis-related septic pulmonary emboli and drug-induced pulmonary edema) and a variety of chronic diseases (e.g., talc granulomatosis and pulmonary hypertension).14
As in the general population, HIV-infected patients who are cigarette smokers are at an increased risk of several respiratory illnesses. Bacterial bronchitis and pneumonia are more common in HIV-infected cigarette smokers than in HIV-infected nonsmokers or former smokers. This is especially true for persons with a CD4+ T-lymphocyte count of less than 200 cells/μL.12 In addition, HIV-infected patients who report a long history of cigarette use may present with manifestations of chronic obstructive pulmonary disease (COPD) as the cause of their symptoms.15 In fact, HIV-infected cigarette smokers appear to be at greater risk for COPD than comparable HIV negative smokers.16 Most cases of lung cancer reported in HIV-infected patients have developed in those persons with a history of cigarette smoking. One can speculate that the incidence of nonHiv-associated pulmonary conditions–many related to cigarette smoking–will increase among HIV-infected patients receiving antiretroviral therapy (ART) as they live longer and as the incidence of HIV-associated OIs and neoplasms and the resulting mortality declines among them. Studies have demonstrated that HIV-positive patients who currently smoke have increased mortality and decreased quality of life, as well as increased respiratory symptoms, compared to nonsmokers.17 As such, increased focus on strategies to reduce cigarette smoking is warranted.
TRAVEL AND RESIDENCE
TB is especially common in certain geographic areas and in certain populations.18,19 HIV-infected patients who were born in or have traveled to a country with a high prevalence of TB and patients who are homeless, unstably housed, or previously incarcerated are at higher risk for exposure to Mycobacterium tuberculosis. Injection drug users, especially if anergic, are another population at increased risk for TB.20 Patients who have a positive tuberculin skin test (defined as a ≥5 mm induration in HIV-infected persons), especially if recent converters, are also at increased risk for developing TB.18,21
PRIOR ILLNESS AND USE OF PROPHYLAXIS
Many HIV-associated OIs are recurrent. Recognition that bacterial pneumonias are common in HIV-infected patients and frequently recur led to the inclusion of recurrent pneumonia (i.e., two or more episodes within a 12-month period) as an AIDS-defining condition in the 1993 Expanded Surveillance Case Definition for AIDS by the US Centers for Disease Control and Prevention (CDC).22 Often patients with multiple, recurrent episodes of severe bacterial pneumonia develop airway damage or bronchiectasis, which in turn predisposes them to further bacterial infections.15 Some such patients develop multidrug-resistant bacteria that are refractory to treatment.
Patients with prior PCP are at high risk for recurrence, and current recommendations advise secondary PCP prophylaxis unless ART has produced a sustained (e.g., >3 months) CD4+ T-lymphocyte count of more than 200 cells/μL.23 Similarly, patients with a history of cryptococcosis, coccidioidomycosis, or histoplasmosis are at high-risk for relapse and should receive chronic maintenance therapy with fluconazole (cryptococcosis and coccidioidomycosis) or itraconazole (histoplasmosis).23 Patients with a significant CD4+ T-lymphocyte count response to ART can discontinue maintenance therapy provided their cell count remains above the threshold associated with an increased risk of disease (e.g., >100–200 cells/μL sustained for >6 months for cryptococcosis).
Chronic antimicrobial use clearly influences the likelihood of the targeted pathogen recurring, but it also affects the likelihood and the drug susceptibility of other organisms causing respiratory disease. For example, at San Francisco General Hospital, the dramatic rise in bacteria resistant to trimethoprim-sulfamethoxazole has coincided with use of trimethoprim-sulfamethoxazole for PCP prophylaxis.24
Current guidelines recommend pneumococcal vaccine and annual influenza immunization.23 Their efficacy is best established in patients with CD4+ T-lymphocyte counts of more than 200–500 cells/μL and is likely to be diminished in patients with low CD4+ T-lymphocyte cell counts. Pneumococcal immunization can be repeated every 5 years and consideration given to repeat immunization for patients who were immunized prior to a rise in CD4+ T-lymphocyte counts >200 cells/μL induced by ART.23 There is also evidence that heptavalent pneumococcal vaccination for children in the household or in the community leads to a reduction in pneumococcal disease among HIV-infected adults. Moreover, community immunization programs lead to change in the serotype of strains infecting HIV-infected adults to nonvaccine strains.25
SYMPTOMS AND SIGNS
The approach to respiratory disease in an HIV-infected patient begins with a thorough history and physical examination. Although nonspecific, constellations of particular symptoms and signs can often point to a specific diagnosis (Table 69-2).
Respiratory symptoms are more common in HIV-infected persons compared to HIV-negative individuals.26 The PCHIS demonstrated that respiratory symptoms are a frequent complaint in HIV-infected persons, and that symptoms increase in frequency as the CD4+ T-lymphocyte count decreases. In this study, subjects reported cough at 27% of more than 12 000 routine visits, dyspnea at 23%, and fever at 9% (Huang, unpublished data). These symptoms increased in frequency in the subset of subjects with a CD4+ T-lymphocyte count of less than 200 cells/μL.
All of the HIV-associated respiratory diseases may present with cough,27 dyspnea, and less frequently pleuritic chest pain. Particular aspects of these symptoms may be especially useful for suggesting a specific diagnosis. Most patients with bacterial bronchitis or pneumonia present with a cough productive of purulent sputum, whereas most patients with PCP note a dry, nonproductive cough.15 In addition to the specific symptoms themselves, the duration of symptoms may also be useful. Bacterial pneumonias due to S. pneumoniae and Haemophilus spp. characteristically present with an acute onset and a symptom duration of 3–5 days. In contrast, PCP usually presents with a subacute, more insidious onset and a typical symptom duration of 2–4 weeks. Kovacs and co-workers found a median symptom duration of 28 days among 49 HIV-infected patients with PCP.28 Thus, in an HIV-infected patient with a CD4+ T-lymphocyte count of less than 200 cells/μL (and hence at risk for both bacterial pneumonia and PCP), the presence of cough productive of purulent sputum of a few days’ duration favors the diagnosis of bacterial infection (Table 69-3). In contrast, a nonproductive cough of a few weeks’ duration strongly favors the diagnosis of PCP.
Table 69-3 Presentation of Bacterial and Pneumocystis Pneumonia
| Parameter | Bacteria | Pneumocystis |
|---|---|---|
| CD4+ T-lymphocyte count | Any | ≤200 cells/μL |
| Symptoms | Fever, chills or rigors | Fever |
| Dyspnea | Dyspnea | |
| Pleuritic chest pain | ||
| Productive cough | Nonproductive cough | |
| Purulent sputum | ||
| Symptom duration | Typically 3–5 days | Typically 2–4 weeks |
| Signs: lung examination | Focal findings | Often unremarkable |
| Inspiratory crackles | ||
| Laboratory tests | WBC frequently elevated | WBC varies |
| LDH varies | LDH frequently elevated | |
| ABG nonspecifica | ABG nonspecifica | |
| Chest radiography | ||
| Distribution | Patchy, focal >multifocal | Diffuse >focal |
| Location | Unilateral, segmental/lobar | Bilateral |
| Pattern | Consolidation | Reticular-granular |
| Associated findings | ||
| Cysts | Rarely | 15–20% |
| Pleural effusions | 25–50% | Very rarely |
| Adenopathy | Rarely | Very rarely |
| Pneumothorax | Rarely | Occasionally |
| Normal radiograph | Never | Occasionally |
ABG, arterial blood gases; LDH, lactate dehydrogenase; WBC, white blood cell count.
a Used to determine severity of disease and whether adjunctive corticosteroids are indicated.
Clinicians should be aware of one caveat: patients may have dual infections (e.g., bacterial infection and PCP). Afessa and colleagues reported that more than 10% of the 111 episodes of bacterial pneumonia reviewed were accompanied by PCP.29 A similar proportion (10%) of patients with PCP present with findings consistent with bacterial co-infection (Fig. 69-1). Although HIV-infected patients can present with multiple concurrent illnesses, extrapulmonary symptoms, when present, are often useful for suggesting a specific, unifying diagnosis, as many of the HIV-associated pulmonary diseases have important extrapulmonary manifestations. For example, the presence of headache in a patient with a CD4+ T-lymphocyte cell count of less than 200 cells/μL and respiratory complaints suggest the possibility of Cryptococcus neoformans meningitis and pneumonia. In fact, although the lungs are the portals of entry for Cryptococcus, many patients present with asymptomatic or minimally symptomatic pulmonary disease, and the diagnosis is only suggested by the presence of extrapulmonary symptoms. In a series of 106 patients with C. neoformans infection, 89 patients (84%) presented with meningitis; cough or dyspnea was present in fewer than one-third of these patients (n = 28, 31%).30 In the National Institute of Allergy and Infectious Diseases Mycoses Study Group and the AIDS Clinical Trials Group (ACTG) study of treatment regimens for cryptococcal meningitis, 31% of the patients randomized to amphotericin B and 35% of those randomized to amphotericin B and flucytosine reported cough; 90% and 89% of the subjects, respectively, reported headache.31
Knowledge of extrapulmonary disease can also be used occasionally to infer the pulmonary diagnosis without specific microbiologic or pathologic confirmation. For example, patients diagnosed with non-Hodgkin lymphoma from a biopsy of a nonpulmonary site who also have findings suggestive of thoracic involvement might potentially receive initial therapy for non-Hodgkin lymphoma and might have specific pulmonary diagnostic procedures reserved for situations where the pulmonary disease progresses despite this therapy.
Patients with bacterial pneumonia often have focal lung examinations suggestive of consolidation, pleural effusion, or both, whereas patients with PCP may have bilateral inspiratory crackles (Table 69-3). Wheezing in a patient with a history of asthma suggests an exacerbation of that condition, whereas diminished breath sounds in a longtime cigarette smoker may indicate emphysema. Absent breath sounds suggest pneumothorax in a patient complaining of pleuritic chest pain, dyspnea, or both. Occasionally, abnormal findings on lung examination are the result of nonpulmonary disease. For example, rales in association with an S3 cardiac gallop and an elevated jugular venous pressure suggest a cardiac etiology for the respiratory complaints.
The remainder of the physical examination may also suggest an etiology for the respiratory symptoms. For example, in a patient whose CD4+ T-lymphocyte count is less than 200 cells/μL, an altered mental status may be secondary to C. neoformans meningitis, whereas a focal neurologic examination may be secondary to Toxoplasma gondii encephalitis. Peripheral lymphadenopathy or hepatosplenomegaly suggests either disseminated mycobacterial or fungal disease or non-Hodgkin lymphoma. New cutaneous lesions may be manifestations of a disseminated fungal disease. The presence of mucocutaneous Kaposi sarcoma lesions may point toward pulmonary involvement with that disease. However, the absence of Kaposi sarcoma lesions on visual examination of the skin and mucous membranes does not preclude the possibility of significant visceral disease, including the lung. Huang and colleagues found that 15% of 168 patients with pulmonary Kaposi sarcoma diagnosed by bronchoscopy had no evidence of concurrent or preexisting mucocutaneous Kaposi sarcoma.32
LABORATORY TESTS
CD4+ T-Lymphocyte Counts
The CD4+ T-lymphocyte count is an excellent indicator of an HIV-infected patient’s risk of developing a specific OI or neoplasm (Table 69-4). The CD4+ T-lymphocyte count is relevant to the occurrence of bacterial pneumonia as well as more traditional OIs. Hirschtick and colleagues in the PCHIS found that the risk of bacterial pneumonia among subjects with a CD4+ T-lymphocyte count of less than 200 cells/μL was more than five and a half times greater than that for subjects whose count was more than 500 cells/μL.12 In addition, as the CD4+ T-lymphocyte count decreases, the incidence of bacterial pneumonia accompanied by bacteremia or septicemia and of M. tuberculosis infection accompanied by extrapulmonary or disseminated disease increase.33,34 Nonspecific interstitial pneumonitis (NSIP), whose clinical presentation and chest radiographic features may be indistinguishable from PCP, differs from PCP in that NSIP may present at a CD4+ T-lymphocyte count of more than 200 cells/μL. In a series reported by Sattler and associates, the mean CD4+ T-lymphocyte count of 16 patients with NSIP was 492 cells/μL.35
Table 69-4 CD4+ T-Lymphocyte Count Ranges for Selected Respiratory Disease
| Any CD4+ T-lymphocyte Count Upper respiratory tract infection Acute bronchitis/sinusitis Asthma Chronic obstructive pulmonary disease Pulmonary arterial hypertension Bacterial pneumonia (most often Streptococcus pneumoniae, Haemophilus spp.) Mycobacterium tuberculosis pneumonia Non-Hodgkin lymphoma Bronchogenic carcinoma Nonspecific interstitial pneumonitis |
| CD4+ T-lymphocyte Count <200 cells/μL Pneumocystis pneumonia Cryptococcus neoformans pneumonia Bacterial pneumonia accompanied by bacteremia or septicemia Extrapulmonary or disseminated M. tuberculosis |
| CD4+ T-lymphocyte Count <100 cells/μL Bacterial pneumonia due to Pseudomonas aeruginosa Toxoplasma gondii pneumonia Pulmonary Kaposi sarcoma |
| CD4+ T-lymphocyte Count <50 cells/μL Histoplasma capsulatum: usually associated with disseminated disease Coccidioides immitis: usually associated with disseminated disease Aspergillus species (most often A. fumigatus) pneumonia Cytomegalovirus pneumonia: usually associated with disseminated disease Mycobacterium avium complex: usually associated with disseminated disease |
At CD4+ T-lymphocyte counts of less than 200 cells/μL, PCP and Cryptococcus neoformans pneumonia become important diagnoses to consider, whereas neither is common in patients with counts that are significantly higher than 200 cells/μL. In a retrospective review of episodes of opportunistic pneumonia diagnosed at the Clinical Center of the National Institutes of Health, 46 of 49 patients (94%) diagnosed with PCP had a CD4+ T-lymphocyte count of less than 200 cells/μL.36 The Multicenter AIDS Cohort Study demonstrated that subjects with a CD4+ T-lymphocyte count of less than 200 cells/μL had a nearly five-fold higher risk of developing PCP than subjects who had a count higher than 200 cells/μL at study entry.37 Stansell and the PCHIS showed that 95% of 145 cases of PCP occurred in subjects whose CD4+ T-lymphocyte count was less than 200 cells/μL (median count 29 cells/μL).38 Darras-Joly et al in a review of 76 patients with cryptococcal disease, found a mean CD4+ T-lymphocyte count of 46 cells/μL (range 2–220 cells/μL) in 65 patients with meningitis.39 The median CD4+ T-lymphocyte counts among 381 patients with cryptococcal meningitis randomized to either amphotericin B alone or amphotericin B with flucytosine for initial treatment were 18 and 20 cells/μL, respectively.31
At CD4+ T-lymphocyte counts of less than 100 cells/μL, pulmonary diseases caused by Pseudomonas aeruginosa, T. gondii, and Kaposi sarcoma are increasingly common. A study of 64 patients with pulmonary toxoplasmosis diagnosed by bronchoalveolar lavage (BAL) reported a mean CD4+ T-lymphocyte count of 40 cells/μL; 82% had a count of less than 50 cells/μL; and only 4% had a count of more than 200 cells/μL.40 One series of 168 consecutive patients with pulmonary Kaposi sarcoma diagnosed by bronchoscopy reported a median CD4+ T-lymphocyte count of 19 cells/μL; 68% had a count below 50 cells/μL, and only 4% had a count above 200 cells/μL.32 Finally, at CD4+ T-lymphocyte counts of less than 50 cells/μL, diseases may be caused by endemic fungi (H. capsulatum, C. immitis) and nonendemic fungi (Aspergillus spp.). Cytomegalovirus (CMV) and nontuberculous mycobacteria (Mycobacterium avium complex) can also cause disease but such cases are quite unusual.
Numerous studies have indicated that the current CD4+ T-lymphocyte count is the best reflection of the risk for a specific OI.23,41 For PCP, several studies have demonstrated that it is safe to discontinue primary or secondary PCP prophylaxis in HIV-infected subjects whose CD4+ T-lymphocyte count has risen from below to above 200 cells/μL.42–49 However, as noted above, cases of PCP are well documented to occur occasionally at CD4+ T-lymphocyte cell counts above 200 cells/μL both in patients who have never received ART, and in patients whose CD4+ T-lymphocyte cell counts have risen from low levels to above 200 cells/μL.50 For example, a patient previously had been able to discontinue treatment successfully for disseminated M. avium complex and disseminated fungal disease without relapse. Yet, despite a CD4+ T-lymphocyte count of 300 cells/μL just prior to the onset of PCP, PCP developed 4 months after secondary PCP prophylaxis was discontinued.50 Thus, although important, the CD4+ T-lymphocyte count should be a guide as to which pulmonary diseases are most common in that population. Exceptions to such guidelines occurred before the era of ART, and exceptions continue to occur.
Arterial Blood Gases
Arterial blood gas (ABG) analysis is indicated for persons with clinical evidence of moderate to severe pulmonary disease (Table 69-2). ABG analysis is also useful for prognosis and for making clinical decisions regarding whether (and where) to admit the patient and whether adjunctive cortic-osteroids are indicated in patients with suspected or confirmed PCP. Laboratory tests that may be useful for suggesting a specific diagnosis include the white blood cell (WBC) count and differential count as part of the complete blood count and the serum lactate dehydrogenase (LDH) assay. Beyond their potential use as part of the diagnostic evaluation, these tests serve as prognostic markers and baseline values for subsequent measurements. Serial measurements are useful in any patient who fails to improve or who worsens despite apparent appropriate therapy.
Serum LDH
Serum LDH is often (but not invariably) elevated in patients with PCP.15 However, LDH may also be elevated in other pulmonary (including bacterial pneumonia and TB) and nonpulmonary conditions, making this test more useful for prognosis than diagnosis. Most of the studies reporting a high sensitivity of serum LDH for PCP consisted of hospitalized patients, some of whom had acute respiratory failure and were mechanically ventilated. The study that reported the lowest sensitivity examined outpatients presenting to a clinic.51 This suggests that PCP severity and the patient population studied affect the diagnostic sensitivity of the test.
The degree to which the serum LDH is elevated has been shown to correlate with prognosis and response to therapy.15 Patients with PCP and an initial markedly elevated serum LDH level or a rising serum LDH level despite PCP treatment have a poor prognosis. Patients with PCP that is responding to treatment have a decline in their LDH toward the normal range.52
IMAGING
Standard Chest Radiography
The characteristic chest radiographic findings for selected HIV-associated pulmonary diseases are presented in Table 69-5. For each disease, the findings from a large series (n >30 cases) are presented as an overview. When no large series has been reported, summary data from smaller series are provided. Differences in the description of radiographic findings and the absence of a standardized approach to interpreting and presenting radiographic data limit the ability to combine studies on a specific pulmonary disease into a single summary table.
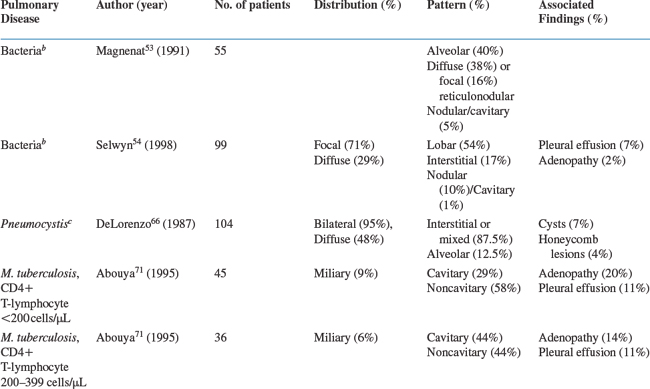
Bacterial pneumonia is the most common pulmonary disease among HIV-infected persons in the United States. The chest radiographic presentation is similar to that of the overall population: focal, segmental, or lobar consolidation. One study reported that of 55 patients with bacterial pneumonia, 40% presented with focal, segmental, or lobar alveolar infiltrates, whereas 38% had diffuse reticulonodular infiltrates, 16% had focal reticulonodular infiltrates, and 5% had nodular or cavitary infiltrates on the chest radiograph.53 Another study of 99 patients found that 54% of the patients with bacterial pneumonia had a lobar infiltrate, 17% had an interstitial infiltrate, 10% had a nodular infiltrate, and only 1% had a cavitary infiltrate on their radiograph. Pleural effusions were seen in 7% and intrathoracic adenopathy in 2%.54 In this study, multivariate analysis demonstrated that the presence of a lobar infiltrate on chest radiograph was independently predictive of bacterial pneumonia. These studies, however, considered all bacterial pathogens together. Numerous reports as well as clinical experience demonstrate that the frequency of specific radiographic findings is dependent on the specific bacteria.
Studies suggest that radiographic presentations may differ among S. pneumoniae, Haemophilus spp., and P. aeruginosa.3,12,55 A review of English-language articles and abstracts from 1981 to 1990 on S. pneumoniae disease in HIV-infected persons found that three-fourths of bacterial pneumonias due to S. pneumoniae presented with segmental, lobar, or multilobar consolidation on chest radiography.56 Garcia-Leoni et al reported that a classic lobar alveolar pattern was seen in 67% and a diffuse alveolar pattern in 10% of 21 patients with S. pneumoniae pneumonia.57 Schlamm and Yancovitz found similar proportions in 34 patients with H. influenzae pneumonia; in this study, focal or diffuse lobar infiltrates were noted in 74%.58 However, another series of 12 patients with H. influenzae pneumonia discovered that the presentation may be clinically and radiographically indistinguishable from that of PCP.59 The patients complained of nonproductive cough and dyspnea with a median symptom duration of 4 weeks. All presented with bilateral interstitial or mixed interstitial-alveolar infiltrates similar to PCP. Cordero et al found that 35% of 26 patients with H. influenzae pneumonia presented with an interstitial pattern on chest radiograph.60 A series of 16 patients with P. aeruginosa pneumonia showed that the pneumonia was community-acquired in 15 patients.61 Chest radiographs revealed cavitary infiltrates on admission in 50%, and an additional 19% presented with pulmonary infiltrates that subsequently cavitated. The frequency of cavitary infiltrates in P. aeruginosa pneumonia was also noted in a study of 25 patients: 24% had cavitary pneumonia.62 Thus the presence of a cavitary pneumonia may be more suggestive of Pseudomonas than either Streptococcus or Haemophilus. However, the differential diagnosis of a pulmonary cavitary lesion is extensive and includes TB, endemic fungal diseases, and Rhodococcus equi.
Pneumonia due to bacteria such as Legionella spp., Mycoplasma pneumoniae, and Chlamydia spp. occurs in HIV-infected patients.63 The frequency of these pathogens as causes of community-acquired pneumonia in HIV-infected patients appears to parallel that of HIV-uninfected patients. A study in Baltimore performed extensive diagnostic testing on 180 HIV-infected patients: Legionella pneumophila (3%), Chlamydia pneumoniae (4%), and M. pneumoniae (<1%) were rarely detected.3 The frequency of these pathogens matched these of the 205 HIV-uninfected patients (3%, 3%, and 1%, respectively) admitted during the same period.
Rhodococcus equi and Nocardia spp. (especially N. asteroides) have been described as causes of pneumonia in HIV-infected patients.64,65 Neither is recognized often.
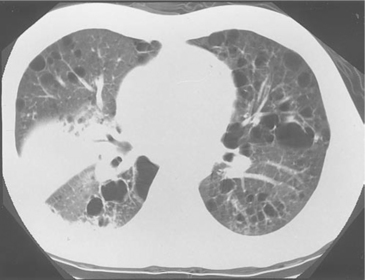
PCP remains a common AIDS-defining OI in the United States. The classic PCP presentation is bilateral interstitial-reticular or granular opacities that are often diffuse. One large study of 104 patients with PCP revealed that 87.5% presented with an interstitial pattern (75.0%) or a mixed interstitial-alveolar pattern (12.5%); the remaining patients had an alveolar pattern.66 In addition, 7% of the radiographs had thin-walled cysts (i.e., pneumatoceles), and 4% had honeycomb lesions. Infiltrates were bilateral in 95% and unilateral in 5%; they involved the entire lung in 48%. This study remains the largest radiology series of patients with PCP, but it predates the widespread use of PCP prophylaxis. Since then, a number of reports have described the radiographic findings in patients receiving aerosolized pentamidine (AP) prophylaxis; these radiographs characteristically reveal an upper lung zone predominance that can mimic mycobacterial disease.67 However, this upper lung zone predominance can also be seen in patients who have never received AP prophylaxis, and the pattern seen (i.e., reticular or granular) is more important than the distribution for suggesting the diagnosis of PCP. In the PCHIS, 467 subjects presenting for evaluation of new or worsening respiratory complaints underwent chest radiography.68 In a multivariate analysis of the 174 subjects with an abnormal radiograph, the presence of interstitial infiltrates on the chest radiograph was an independent predictor of PCP.
TB can present with a variety of chest radiographic findings, including upper lung-zone infiltrates often with cavitation, middle or lower lung zone consolidation mimicking bacterial pneumonia, miliary disease, nodule(s), pleural effusions, and intrathoracic adenopathy. TB may also present with a normal chest radiograph. The frequency of these radiographic findings is influenced by the patient’s CD4+ T-lymphocyte count.69 Jones and colleagues reported that mediastinal adenopathy was found in 13% of the 30 patients with a CD4+ T-lymphocyte count higher than 200 cells/μL compared with 36% of the 58 patients with a count less than 200 cells/μL (P = 0.02).70 Abouya and coworkers reviewed the chest radiographic presentation of 111 HIV-infected patients with TB. The proportion of patients with cavitary infiltrates decreased as the CD4+ T-lymphocyte count decreased from more than 400 cells/μL to 200 to 399 cells/μL, and then to less than 200 cells/μL (P < 0.05), whereas the proportions with noncavitary infiltrates and intrathoracic adenopathy increased as the count decreased (both P < 0.05).71 Perlman and colleagues in the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) and the ACTG pooled data from 128 patients with culture-positive TB.72 When they combined their data with those from published studies, the authors found a significant association between the presence of infiltrates, cavitation (both more likely in patients with a CD4+ T-lymphocyte count of 200 cells/μL or more), and intrathoracic adenopathy (more likely in patients with a count of less than 200 cells/μL). Thus, the radiographic key to the diagnosis of pulmonary TB is knowledge of the patient’s CD4+ T-lymphocyte count and an understanding of which patterns are more common at that count.
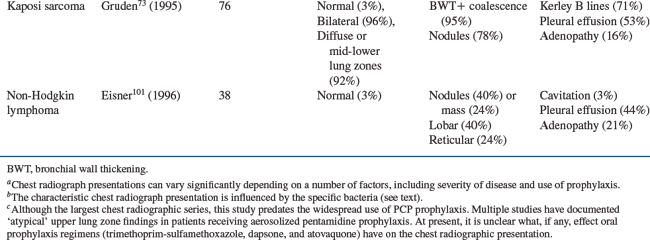
Pulmonary Kaposi sarcoma characteristically presents with bilateral opacities in a central or perihilar distribution and a middle/lower lung zone predominance. Linear densities, nodules, and pleural effusions are all common. In a study of 76 patients with pulmonary Kaposi sarcoma, 95% of the chest radiographs had peribronchial cuffing and tram track opacities or had extensive perihilar coalescent opacities.73 Small nodules (50%) or nodular opacities (28%) were seen in 78%, Kerley B lines in 71%, and pleural effusions in 53% of the radiographs. No patient presented with either Kerley B lines or pleural effusions without concurrent parenchymal findings. Sixteen percent of these patients had hilar or mediastinal lymph node enlargement.
Chest Computed Tomography
For most evaluations of symptomatic HIV-infected patients, a computed tomography (CT) scan is unnecessary because the clinical and chest radiographic presentation suggests a single diagnosis or a few diagnoses to consider.74,75 However, a chest high-resolution CT (HRCT) is extremely useful in cases of clinically suspected PCP in which the chest radiograph is normal or unchanged, a phenomenon that occurred in 39% of one reported series.76 Most patients with respiratory symptoms suggestive of PCP whose radiograph is normal or unchanged do not have PCP. Subjecting these patients to diagnostic procedures such as bronchoscopy or to empiric PCP treatment with its associated toxicities is ill-advised. In these cases, a sensitive test is needed to select which patients require diagnostic procedures or empiric therapy and, just as important, which patients can be observed without either of the above. The chest HRCT scan is one of several such tests. Patients with PCP and a normal chest radiograph have patchy areas of ground-glass opacities (GGOs) on HRCT.74,75 Although the presence of GGOs is nonspecific and may be seen with a number of pulmonary disorders, its absence strongly argues against the presence of PCP. In one study, none of the 40 HIV-infected patients with clinically suspected PCP, a normal or nonspecific chest radiograph, and an HRCT without GGOs had PCP diagnosed by BAL fluid examination or 60 days of clinical follow-up.77 In fact, no patient with a normal chest radiograph and an HRCT without GGOs has been diagnosed with PCP at San Francisco General Hospital (Laurence Huang, personal observation).
Chest CT can also be useful for suggesting a diagnosis in cases in which the chest radiograph reveals multiple pulmonary nodules. A predominance of nodules smaller than 1 cm in diameter in a centrilobular distribution strongly suggests the presence of an OI, whereas a predominance of nodules larger than 1 cm in diameter is suggestive of a neoplasm.78,79 In cases where the nodules are mostly smaller than 1 cm in diameter, the presence of intrathoracic adenopathy, especially if low attenuation (another potential use for CT) indicates that mycobacterial (or fungal) disease is probable.80 In cases in which the nodules are mostly larger than 1 cm, the finding of associated peribronchovascular thickening inevitably results in a diagnosis of pulmonary Kaposi sarcoma. Finally, chest CT scans are useful for guiding diagnostic procedures such as bronchoscopy, CT-guided transthoracic needle aspiration, and surgical procedures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree