23.1
Introduction
Risk of osteoporosis and fracture in older women is, in large part, related to the woman’s bone mineral density (BMD) ; however, the predisposition to osteoporosis and fracture may be established by the level of young adulthood peak bone mass. Stochastic models developed by Horsman and Burkinshaw suggested that two-thirds of the risk for fracture can be predicted based on premenopausal BMD. Therefore in premenopausal women, the primary goal is to maximize or maintain BMD. The World Health Organization (WHO) characterizes osteoporosis based on normative data from women aged 20–40 years. By the WHO definition, osteoporosis exists when BMD is 2.5 standard deviations (SDs) below the mean values for women aged 20–40 years. Greater acquisition and longer maintenance of premenopausal bone mass can establish a bone mineral reserve that could ultimately reduce the risk for osteoporosis and fracture following menopause. Identifying those factors related to the accrual, maintenance, or diminution of bone is important given the difficulty in restoring lost bone.
Reproductive activities and the hormones associated with reproduction may play a central role in determining BMD levels during pre- and perimenopause. In this chapter, those endogenous and exogenous events that are related directly or indirectly to the capacity to reproduce are considered for their importance to peak bone mass. In particular, this chapter includes updated information about bone loss in pregnancy, the importance of injectable and oral contraceptives on peak bone mass acquisition, liberation of heavy metals from the bone depot during pregnancy and lactation, and studies of luteal functioning and bone (see also Chapter 38: Osteoporosis in premenopausal women, pregnancy, and lactation ).
23.2
Pregnancy
Pregnancy and lactation are characterized by alterations in the maternal hormone environment, notably estrogen and prolactin concentrations. During the third trimester of pregnancy, estrogen levels rise as the placenta contributes to large quantities of estriol . In marked contrast, lactation represents a hypoestrogenic state with elevated prolactin concentrations . These events are associated with a substantial calcium transfer from the mother for redistribution to the fetus or infant. The total accumulation of calcium in a full-term neonate during pregnancy is approximately 30 g . If maternal bone was the sole source of calcium, the mother’s skeleton would lose about 3% (30/1000 g) of its mineral per pregnancy.
It has been unclear whether the size of the maternal bone depot is reduced during pregnancy. Typically, less than 20%–30% of ingested dietary calcium is actually absorbed in an adult woman, and the remaining calcium is excreted in the feces. If absorption efficiency were doubled from 20% to 40% in women consuming moderate calcium intakes, the skeletal needs of the fetus could be met without extensively accessing the mineral stored in the maternal skeleton. Likewise, reducing maternal urinary calcium excretion could potentially also allow the demands of the mother and child to be met without an impact on the size of the maternal bone depot.
A number of metabolic adaptations take place early in pregnancy to address the mineralization demands of the fetus, including an increase in intestinal calcium absorptive capacity in response to an oral calcium load ; a slowing of gastric motility; an increase in renal resorption; an increase in the extracellular fluid volume; an increase in urinary calcium excretion ; and a modest decline in serum calcium concentrations in the second trimester , apparently in parallel with the decline in serum albumin . This collective response would appear to preclude negative calcium balance even in adolescent pregnant women where the calcium needs to support fetal and maternal growth must be addressed.
23.2.1
Studies of bone mass and pregnancy
Until recently, studies of bone mass and pregnancy suggested either no measurable bone mass loss with pregnancy or bone loss in specific compartments (trabecular rather than cortical) or only at selected bone sites . However, the growing availability and validation of bone ultrasound technology has changed our understanding of bone loss with pregnancy . Studies by Aguado et al. , Sowers et al. , Gambacciani et al. , Tranquilli et al. , and Hellmeyer et al. have all reported a modestly lower maternal bone mass with pregnancy, although an ultrasound-based study by Yamaga et al. did not confirm this among Japanese women. These studies addressed many of the assessment issues associated with the use of dual-energy X-ray absorptiometry (DXA) densitometry during pregnancy. A net deficit in bone calcium balance, occurring during both pregnancy and early lactation, has been described with kinetics studies .
Early studies of DXA and pregnancy suffered from small sample sizes that lacked sufficient power to detect a 3%–4% difference in bone mass change that might be expected during a pregnancy, but two subsequent studies describe loss, then recovery . Olausson et al. evaluated 34 women prior to pregnancy and immediately postpartum and noted a 1% and 4% decrease in bone mineral content (BMC), areal BMD, and bone area-adjusted BMC at the whole body, spine, and total hip. Møller et al. measured BMD and body composition in 153 women in prepregnancy, in each trimester and four times postpartum, noting similar loss during pregnancy and further loss with breastfeeding, but full recovery by 19 months postpartum. More recently, Wei et al. confirmed BMD and BMC loss by DXA at both spine and femoral neck from 12–20 weeks gestation to 0–14 weeks postpartum in 301 African-American, Caucasian, and Hispanic women, with African-American woman losing more spine BMD than Caucasians . Studies had not addressed the issue of age (adolescent pregnancy or pregnancy at obstetric maturity) and the potential for women in these groups to have different calcium needs and a different responsiveness of bone to the calcium demand of pregnancy on bone.
23.2.2
Studies of bone and pregnancy using biochemical markers
The most frequently characterized bone turnover markers measured in pregnancy include circulating osteocalcin and alkaline phosphatase concentrations as indicators of bone formation. Until recently, markers of formation and resorption typically have not been reported simultaneously to more fully characterize the bone turnover experience. In a study of Italian women, it was reported that the resorption markers pyridoline and deoxypyridoline were lower in pregnant women as compared to controls .
Serum concentrations of osteocalcin tend to be comparable to control values in the first trimester, decline in the second trimester of the pregnancy, and then recover in the third trimester to levels observed in normal nonpregnant controls. This has been observed in studies with repeated measures or static comparisons . However, Møller et al. reported a decrease in osteocalcin in the first trimester compared to prepregnancy levels with a further decline in the second trimester and recovery to control levels in the third trimester in 92 women through 9 months of lactation who prospectively conceived . Osteocalcin levels were then increased through 9 months postpartum regardless of breastfeeding status. Rodin et al. observed that osteocalcin concentrations were within normal range within 48 hours of delivery. Notably, Sowers et al. reported that the association of osteocalcin and insulin-like growth factor I (IGF-I) in local bone regulation is different in women who are normotensive as compared to preeclamptic, so different turnover marker associations may be present depending on maternal health during pregnancy.
Like osteocalcin, alkaline phosphatase has been evaluated as a marker of bone formation during pregnancy. Total serum alkaline phosphatase activity (ALK) increases gradually in the first and second trimesters, with a rapid increase in the third trimester . Rodin et al. reported that both placental and bone-specific alkaline phosphatase isoenzyme patterns replicate the pattern seen in total alkaline phosphatase concentrations during pregnancy. In addition, they documented that placental ALK declines to levels observed in the first trimester by 6 months postpartum; however, both total and bone-specific alkaline phosphatase are elevated at 6 weeks postpartum. In women who are lactating, activity remains elevated. Hellmeyer et al. followed bone resorption markers, serum type I collagen C-telopeptides (CTX) and a cross-linked peptide of the carboxy-terminal telopeptide of type I collagen (ICPT) and formation markers, bone alkaline phosphatase (BAP) and the N-terminal propeptides of type I collagen (PINP), and noted a significant increase in all markers over the course of pregnancy . More recently, Møller et al. reported a similar increase in urinary cross-linked N-terminal telopeptide of type 1 collagen, indexed to creatinine (NTx/Cr), throughout pregnancy but a contrasting decrease in BAP in the first and second trimester followed by recovery in the third trimester and an increase during breastfeeding .
There has been only a preliminary examination of the role of the receptor activator of nuclear factor-κB ligand (RANKL) pathway in reproduction. These studies need to incorporate osteoprotegerin (OPG), an osteoblast-derived protein that binds to the RANKL, a member of the RANKL signaling pathway that regulates osteoclastogenesis and osteoclast activation . One study indicates that there was no association of OPG with bone turnover and BMD during pregnancy, although the report was limited to data from 17 women .
23.2.3
Studies of pregnancy and calciotropic hormones
The numerous studies of calciotropic hormones [parathyroid hormone (PTH), 1,25-dihydroxyvitamin D, and calcitonin] during pregnancy have been reviewed by Verhaeghe and Bouillon , Chesney et al. , Sowers , and Kovacs and Kronenberg .
PTH promotes increased calcium mobilization from bone in response to lower levels of circulating calcium concentrations. Initially, pregnancy was regarded as a state of “physiologic hyperparathyroidism,” as pregnancy was associated with an increase in PTH concentrations. More recent studies, using more specific assays, challenged this concept and have reported either no significant elevation of PTH with gestation or a decrease in PTH , relative to nonpregnant controls. The studies have generally not addressed dietary calcium intake, vitamin D status, or other factors that could, theoretically, influence PTH secretion.
While the concept of “physiologic hyperparathyroidism” has been eclipsed, there is still the potential for functional hyperparathyroidism to exist in the absence of elevated PTH levels. Another agent, parathyroid-related peptide (PTH-rP), with sequence homology similar to PTH, has been described as being higher in pregnant women as compared to nonpregnant controls. In pregnancy, PTH-rP appears to play multiple roles, including promoting maternal–fetal calcium transfer and milk production .
It is recognized that adequate vitamin D concentrations are necessary during pregnancy. Adequacy of vitamin D has been a source of concern, not so much for its association with bone loss, but because of the potential association with neonatal tetany. A recent review concludes that the evidence is contradictory (except for maternal infectious disease) and thus inconclusive that maternal vitamin D status influences maternal, fetal, and breastfed infant bone health, and maternal (preeclampsia, gestational diabetes, obstructed labor, and infectious disease), fetal (growth, gestational age, and developmental programing), and infant adverse outcomes .
During pregnancy, 1,25-dihydroxyvitamin D concentrations rise and are believed to be responsible for the enhanced absorption of dietary calcium . Those factors that regulate the hormone during pregnancy are uncertain, although PTH, growth hormone, prolactin, and estrogen have all been suggested as candidates . It is not known whether the increased 1,25-dihydroxyvitamin D levels arise from the placenta. Alterations in the levels of 1,25-dihydroxyvitamin D are not associated with a similar pattern in the levels of 25-hydroxyvitamin D . Investigators have observed that seasonal patterns in 25-hydroxyvitamin D levels in pregnant women are similar to those reported in nonpregnant women .
While it might be hypothesized that calcitonin concentrations should rise during pregnancy to protect the maternal skeleton from resorption, findings from the few studies of calcitonin concentrations during pregnancy have been inconsistent. For example, Stevenson et al. and Whitehead et al. , in cross-sectional studies, reported an increase in calcitonin in pregnant versus nonpregnant women. Pitkin et al. reported at least six different calcitonin patterns when multiple measures were made on study participants. Stevenson et al. observed no difference in calcitonin values between pregnant and lactating women. More recently, Møller et al. reported decreased calcitonin levels throughout pregnancy with normalization following delivery and through 9 months postpartum regardless of nursing status . Synthesis of this information is difficult in that markedly different assays were used in these studies, limiting comparability. In addition, there is now question as to how these assays relate to currently available and more specific calcitonin assays.
23.2.4
Bone lead and bone resorption during pregnancy
There is concern that lead that accumulates in bone may be liberated during pregnancy and lactation, leading to adverse reproductive outcomes and impaired fetal development . Reportedly, there were greater odds of having third trimester hypertension with higher circulating blood lead levels, although this was not observed with higher bone lead levels . There is apparently no maternal–fetal barrier to lead .
In an adult, more than 90% of lead is deposited in bone , where it has a long half-life . Indeed, Gulson et al. reported the use of blood metal lead concentrations and lead isotopic composition to measure lead mobilization and confirm increased bone resorption throughout pregnancy and the postpartum period irrespective of breastfeeding status . Thus, while legislative efforts to reduce lead emissions from combustion engines have led to remarkable reductions in mean blood lead levels , public health measures have not been universally implemented, and there is differential impact of lead exposures on population groups, including children and the poor . This, in combination with the long half-life of lead as well as other heavy metals such as cadmium in bone, has elevated the need for a better understanding of calcium dynamics and heavy metal exposures to new levels.
A number of studies of bone and lead have taken place in Mexico City, where major exposures come from the use of leaded ceramics or result from breathing leaded gasoline emissions . In studying Mexican women, Téllez-Rojo et al. observed that plasma lead concentrations increased during pregnancy with greater bone turnover, as assessed by N-telopeptides, and higher bone lead levels, as assessed with K X-ray fluorescence.
Among 367 breastfeeding women living in Mexico City, the highest breast milk lead levels were reported among those women who were exclusively breastfeeding and had high patellar bone levels , which is consistent with an earlier report among six lactating women where blood lead levels in breastfeeding women continued to rise, reaching maximum levels about 6–8 months following delivery . Manton et al. also reported that levels dropped from one pregnancy to the next . Based on these observations, both research groups have suggested that adequate intakes of calcium may be among the effective public health measures to minimize lead liberation from bone during breastfeeding. Manton et al. suggested that a daily intake of 1000 mg during pregnancy may protect the skeleton from excessive lead resorption in late pregnancy . There is some evidence that higher calcium intakes may afford some protection against lead exposures . This apparent protection may be more relevant in those settings with high lead exposures such as residence adjoining a smelter . Hernandez-Avila et al. reported that among breastfeeding women of Mexico City with higher lead burden, a calcium supplement of 1200 mg of CaCO 3 was associated with a modest reduction in circulating blood levels, subsequently reported to average 11% and to be most evident in the second trimester .
23.2.5
Summary and implications
Initial studies of bone change in pregnancy did not provide evidence of bone loss with pregnancy, although many of these studies had important design limitations. Recent studies of pregnant women using bone ultrasonography, DXA, and bone turnover markers suggest that there is higher bone turnover and loss after 20 weeks of pregnancy. This occurs although fetal demand for mineralization of the skeleton is not particularly high (30 g), and there are adaptive mechanisms, including higher circulating levels of 1,25-dihydroxyvitamin D and increased intestinal absorption efficiency occurring simultaneously during pregnancy. This greater appreciation of bone turnover during pregnancy has motivated evaluation of the impact of bone turnover on the liberation of heavy metals, particularly lead, the presence of which in the circulation may impact reproductive outcomes. Evidence continues to accrue that high lead levels in bone are accompanied by higher circulating lead levels, but these circulating levels might be modestly reduced through the use of calcium supplements.
23.3
Age at first pregnancy
Excess bone resorption with pregnancy may not be a characteristic of the mature woman who has achieved full maximal bone mass. Earlier evidence shows that pregnancy at an earlier age, when the skeleton of both fetus and mother are maturing simultaneously, may result in lower bone density and increased risk for perimenopausal bone loss. More recent data have called that into question.
Sowers et al. observed cross-sectionally that a first pregnancy during adolescence was associated with lower premenopausal radial BMD. A subsequent longitudinal study showed that parous women whose first pregnancy was before age 20 had significantly lower age-adjusted baseline radial BMD, lower follow-up radial BMD, and greater 5-year radial BMD loss. The observation was confirmed by Fox et al. in a cross-sectional study of about 1800 elderly women. The investigators speculated that the hormonal events of pregnancy during adolescence may jeopardize achieving the maximal peak in bone mineralization. Cho et al. noted similar findings in Korean women in a cross-sectional survey of postmenopausal women , and Schnatz et al. reported that both early pregnancy and no history of breastfeeding were associated with postmenopausal osteoporosis . In contrast, more recently Teerapornpuntakit et al. reported slightly increased L1–L2 BMD and no difference in hip BMD in 20 women age 24–30 who had pregnancies and breastfed at age 15–19 compared to 30 nulliparous controls , and Yüce et al. noted higher femoral neck and comparable lumbar spine BMD in 87 perimenopausal Turkish woman with a reported history of adolescent pregnancy compared to 153 controls .
23.4
Parity and nulliparity
The relationship of parity to bone mass is complex and poorly defined. Theoretically, bone mass may decrease because of the calcium demand of pregnancy. In contrast, bone mass may increase with the greater circulating estrogen levels in the third trimester of pregnancy and because of the increased bone loading that occurs with the weight increases in pregnancy. With the uncertain impact of parity on bone mass, it is a logical extension that the impact of parity on fracture is also ill-defined.
23.4.1
Studies of parity and bone mass
A number of studies have reported an increase in bone mass with parity, as measured with different technologies at different bone sites with different parity classifications . Other studies have found no association in studies of premenopausal or postmenopausal women . For example, in studies of Caucasian and Bantu women, Walker et al. found no difference in metacarpal cortical area of women, aged 30–44, who had zero to one child as compared to those with more than six children. Likewise, Kritz-Silverstein et al. reported no association with increasing number of pregnancies in women aged 60–89 years. Hreshchyshyn et al. reported that BMD of the femoral neck declined with increasing number of live births, whereas there was no change in the lumbar spine. Henderson et al. reported similar BMD levels in controls and women who had between 10 and 18 pregnancies and breastfed almost continuously in the interval between pregnancies. Allali et al. noted decreased lumbar and total hip BMD with increased parity in a cross-sectional study of postmenopausal Moroccan women, but no association with peripheral fracture rates . Lenora et al. in a Sri Lankan population and Turan in Turkish women found no cross-sectional association of multiparity or prolonged breastfeeding with BMD at any site in postmenopausal women . From the longitudinal Study of Women’s Health Across the Nation (SWAN), Mori et al. reported that lifetime parity was associated positively with femoral neck strength relative to load but did not affect fracture risk after age 42 over a 16-year follow-up . Cooke-Hubley et al. noted from the Canadian Multicentre Osteoporosis Study (CaMos) in 3437 women who completed 15 years of follow-up that increasing parity had no relationship with spine, total hip, or femoral neck BMD .
Studies of parity and bone mass may have inconsistent findings because at least three factors may differ from one study population to another. These include differences in the ability to conceive, differences in the ability to maintain a viable fetus to term, and differences in the amount of weight gained during and subsequently retained following pregnancy. Successful conception and pregnancy require distinct hormonal environments. To conceive, the hormonal environment must be sufficiently competent to allow the preparation of the endometrial bed and development of the ovum. Bone mass measured in nulliparous women may not be the appropriate comparison to bone mass in parous women. Nulliparous women include those who lack reproductive competence, those who do not have the opportunity to conceive, and those who do not want to conceive. The lack of reproductive competence may be related to lower bone mass. Likewise, among those who do not want to conceive, the use of selected contraceptive preparations may be associated with lower bone mass, particularly if their use was begun during adolescence and prior to reaching peak bone mineralization.
Studies of bone density and fractures in nulliparous women reinforce the concern that they are inappropriate controls for studies of parity and bone. In a longitudinal study of premenopausal women, Sowers et al. found that nulliparity was highly predictive of reduced radial BMD, but not rate of change after controlling for age and body size. There was no relation between the number of children and radial BMD when nulliparous women were not used as the referent group. Fox et al. also identified that nulliparous women had significantly lower bone density of the distal radius among the postmenopausal women enrolled in the Baltimore Center of the Study of Osteoporotic Fractures. The lower radial BMD in nulliparous women suggests that their risk may be associated with an inability to conceive or maintain a pregnancy. Petersen et al. reported an increased risk of fracture with nulliparity compared to having at least one child . As such, careful interpretation of parity data is required if nulliparous women are an integral part of the reference population. Evaluation of parity in future studies should also include adjustment for confounders such as age of the mother and change in weight over time.
23.4.2
Studies of parity and fracture
A longitudinal study and a case–control study provide evidence of a protective effect for parity in relation to hip fracture. In both studies, women with three or more children had an approximate 30%–40% reduction in risk for fracture as compared to nulliparous women. While both studies addressed the contribution of other major potential confounders, the comparison groups, in both instances, were nulliparous women, groups whose biology may carry an intrinsic risk for low bone mass. If nulliparous women have lower BMD, they are likely to have a greater risk of fracture, and a parous group using them as a reference would appear to have an inappropriately reduced risk for fracture. A third study has identified a very modest protective effect of parity for hip fracture, but only among women who had not used oral contraceptives .
Numerous studies have shown no association of parity with fracture. The studies, in widely diverse populations, include hospital-based case–control studies in Connecticut and Toronto ; a population-based case–control study in Seattle, Washington ; a case–control study of older women in southwest France ; a population-based case–control study in Australia ; a cross-sectional survey of postmenopausal women in Morroco ; and longitudinal studies in both SWAN and CaMos .
23.4.3
Summary and implications
It appears that, if there is a protective effect of parity against fractures mediated through greater bone mass, this effect is weak. A stronger case for a protective effect could be made for parity if the studies of both bone mass and fractures had used women with a single pregnancy as the comparison group and evaluated the likelihood of a “dose response” with succeeding numbers of children. The preponderance of evidence of these studies suggests that parity is neutral with respect to its impact on peak bone mass.
23.5
Lactation
23.5.1
Calcium demand and ovarian suppression by lactation
At least two events that occur during lactation may have an impact on bone mass, including increased calcium demand and suppression of the hypothalamic–pituitary–ovarian (HPO) axis. There is substantial potential for significant calcium demand from the maternal skeleton. Mobilization of calcium from the maternal skeleton will be more highly variable than maternal skeletal mobilization in pregnancy, if it occurs, and the degree of calcium mobilization is dependent on the amount of breast milk produced and on the duration of the lactation period. An estimated cost to the maternal skeleton with 6 months of full lactation would be approximately 4%–6% if no compensatory mechanism(s) existed for increasing calcium availability apart from mobilization of the skeletal depot.
Calcium is transferred directly from serum to breast milk. It is estimated that approximately 600 mL/day of milk is produced at 3 months following parturition (168 mg calcium/day) and 1 L of milk is produced per day at 6 months following parturition (280 mg calcium/day). The calcium concentration of milk is regulated and appears to be somewhat constant even in the face of variable maternal calcium intake. However, there is some debate as to the potential for lower calcium content of breast milk in women with very low calcium intakes, as evidenced when West African women were compared to British women . It was initially assumed that there was an increased efficiency of calcium absorption in lactation, parallel to that observed in pregnancy. However, several studies, but not all , have reported that lactation is not associated with increased absorption efficiency .
In addition to the calcium demand with lactation, the hypothalamic–pituitary axis is suppressed in breastfeeding, as evidenced by the lack of luteinizing hormone release following administration of an estrogen challenge to lactating women . Elevated prolactin concentrations associated with lactation inhibit pulsatile pituitary gonadotropic hormone secretion, suppress the positive feedback effects of estrogens, interfere with ovarian steroidogenesis, and induce ovarian refractoriness to gonadotropic stimulation . Women with prolactin-secreting adenomas also illustrate the negative impact of nonlactational-elevated prolactin on bone demineralization .
23.5.2
Studies of bone mass and lactation
Studies of bone mass published between 1960 and 1990 were mixed with respect to the impact of lactation. Various studies suggested bone loss with lactation, no significant negative effect of lactation on subsequent bone mass or fractures, and even a rise in bone density with lactation. However, findings from longitudinal studies and clinical trials have consistently shown significant early losses of BMD at the spine and hip in amounts of 5%–7% of the total BMD . The findings are also reported in animal studies . Importantly, however, several of these studies have also documented that the BMD is largely restored in the 6- to 12-month period following weaning, as menses are reestablished . Sowers et al. reported that women who have lost bone mass during lactation appear to continue recovery during a subsequent pregnancy occurring within 18 months of the previous pregnancy. Further, lower BMD was not identified among women who continued to breastfeed during interpregnancy intervals that included 10–18 pregnancies and live births .
These changes in calcium homeostasis appear to be independent of lifestyle, including dietary calcium intake and exercise. Bone loss and recovery experiences have been reported to occur in Gambian women with low calcium intakes as well as in groups of White women with greater calcium intakes . In addition, Little and Clapp reported that regular, self-selected, and recreational exercise has no impact on early postpartum lactation-induced BMD loss. However, Lovelady et al. demonstrated that a program of postpartum aerobic and resistance exercise reduces lumbar spine BMD and lean body mass loss during lactation .
Caird et al. reported that the bone loss of lactation is somewhat minimized by the use of progestogen-only contraception. Nonetheless, biochemical marker concentrations measured in women using the progestogen closely resemble those observed in lactating women using barrier contraceptive methods.
The mechanism(s) that mediates rapid bone turnover and mobilization of calcium from the maternal skeleton to breast milk is controversial. At least two possible mechanisms may increase skeletal turnover in lactation. The calciotropic hormones, PTH and 1,25-dihydroxyvitamin D, stimulate bone resorption. Thus it was believed that the transfer of calcium and phosphate to breast milk would stimulate PTH and 1,25-dihydroxyvitamin D–induced bone resorption. However, these actions have not been well substantiated in studies of lactation. Indeed, in the rat, it has been reported that the bone loss of lactation is independent of both PTH and vitamin D concentrations . In humans, studies have frequently observed little difference in concentrations of these calciotropic hormones between lactating women and controls , but Carneiro et al. have shown an increase in both C-telopeptide of type I collagen and amino-terminal telopeptides of procollagen I during lactation, suggesting a coupled increase in osteoclast–osteoblast activity . In mice, weaning induces an increase in serum calcium, decrease in PTH, and selective apoptosis of osteoclasts mediated through decreased levels of RANKL leading to rapid recovery of bone mass.
The changes in BMD with lactation appear to be determined by the combined effects of lower estradiol concentrations and higher PTH-rP that may be linked with the higher prolactin concentrations . Data suggested that the changes in calcium homeostasis during lactation are not related to PTH, 1,25-dihydroxyvitamin D, or 25-hydroxyvitamin D concentrations or to the changes in the concentrations of these calciotropic hormones in the postpartum period .
Several lines of evidence suggest that PTH-rP has a significant role in calcium metabolism in lactation. First, PTH-rP was identified initially as the factor associated with the humoral hypercalcemia of malignancy that is expressed in multiple cancer types, but most notably with breast tumors . Second, in animal studies, PTH-rP has been shown to be synthesized in lactating mammary tissue ; in rats, a temporal relationship exists between elevations in serum prolactin levels and the local expression of PTH-rP mRNA levels . High concentrations of PTH-rP have been described in the milk of a variety of mammals . In mice, the calcium-sensing receptor is expressed on mammary epithelial cells and regulates PTH-rP production and calcium transport in lactating mammary glands . But, in mice with a conditional knockout of PTH-rP in pre-osteoblasts and osteoblasts, there is no difference in lumbar spine BMC loss or recovery with lactation and subsequent weaning , suggesting that alternate mechanisms exist.
Sowers et al. found that elevated PTH-rP concentrations were significantly associated with breastfeeding status, elevated prolactin levels, and lower estradiol levels, all conditions related functionally or endocrinologically to lactation. As shown with P -values in Table 23.1 , PTH-rP was the consistent and significant predictor in all four of the femoral neck BMD change models and three of the four longitudinal models for lumbar spine change, independent of the inclusion of serum prolactin or estradiol concentrations, time since resumption of menses, or breastfeeding practice. Furthermore, PTH-rP values were associated negatively and significantly with BMD change in the spine and femoral neck over time. The primary role of PTH-rP in calcium metabolism during lactation may be more prominent in the early months following parturition. Consistent with a linkage of greater prolactin and detectable PTH-rP values is the report by Stiegler et al. that detectable concentrations of PTH-rP were observed in approximately 50% of men and women with prolactin-secreting adenomas and osteopenia.
| Breastfeeding practice | |||||||
|---|---|---|---|---|---|---|---|
| Model | Time (months) | PTH-rP (pmol/L) | Prolactin (ng/mL) | Fully | Partially | Estradiol (pg/mL) | Menses resume |
| Spine | |||||||
| I | (0.001) | (0.01) | (0.001) | – | – | (0.001) | – |
| II | (0.01) | (0.18) | – | (0.001) | (0.97) | (0.001) | – |
| III | (0.02) | (0.01) | (0.001) | – | – | – | (0.001) |
| IV | (0.06) | (0.03) | – | (0.001) | (0.15) | – | (0.01) |
| Femoral neck | |||||||
| I | (0.06) | (0.02) | (0.01) | – | – | (0.18) | – |
| II | (0.04) | (0.01) | – | (0.01) | (0.01) | (0.26) | – |
| III | (0.08) | (0.03) | (0.03) | – | – | – | (0.18) |
| IV | (0.08) | (0.01) | – | (0.01) | (0.68) | – | (0.06) |
The transitory elevation in PTH-rP concentrations as women initiate weaning might even contribute to the BMD recovery observed between 6 and 18 months following parturition. Using tissue culture systems of fetal rat calvariae, Canalis et al. demonstrated that continuous treatment with PTH-rP reduced labeled proline incorporation into bone collagen by 50%. However, transient exposure to PTH-rP actually doubled the increase in proline incorporation, an effect that the investigators attributed to enhancement of the local production of IGF-I. Early in lactation, more constant PTH-rP concentrations may be sustained by more frequent suckling. These sustained PTH-rP concentrations, in turn, may minimize the amount of bone collagen formation and stimulate both bone resorption and formation. These actions would tend to assure a source of calcium and phosphate for incorporation into breast milk. Likewise, as lactation frequency subsides or as weaning is introduced, PTH-rP secretion would become more episodic. Using as a paradigm the Canalis data as well as similar findings in studies conducted with PTH , one could speculate that bone mineral equilibrium would be restored and then measured as bone mass recovery. The multiple lines of evidence and the temporality provide a compelling argument for a biological role for PTH-rP in calcium transfer during lactation.
While there appears to be bone loss and bone mineral recovery with extended lactation, unanswered questions still remain. The mechanisms by which loss and recovery occur need further elucidation. Studies are needed in specific subgroups, including adolescents, women of obstetric maturity who are lactating, and women with extended and repeated lactation.
23.5.3
Studies of lactation and fracture
The likelihood that lactation is associated with subsequent fracture risk appears to be influenced by the duration of the lactation and by whether the comparison group is based on parous or nulliparous women . For example, a case–control study investigating risk factors for fracture in postmenopausal women found no overall greater fracture risk in women who had breastfed versus women who had never breastfed . However, stratified analysis suggested that breastfeeding for less than 1 year might increase the risk, whereas breastfeeding in excess of 1 year might decrease the risk. The case–control study by Kreiger et al. suggested a protective effect for breastfeeding. Large longitudinal studies suggest no increased fracture risk associated with lactation. Mori et al. reported that, in SWAN, in multiple linear regressions adjusted for covariates, accumulated length of lactation was associated negatively with lumbar spine BMD, while in Cox proportional hazards regressions adjusted for covariates, neither parity nor lactation was associated with fracture hazard after age 42 over a 16-year follow-up . Cooke-Hubley et al. noted from CaMos in 3437 women over 15 years of follow-up that breastfeeding duration had no relationship with incident major fragility fractures .
The importance of comparison group definition is demonstrated in the data of Hoffman et al. as well as Cumming and Klineberg . Hoffman et al. reported a protective effect of breastfeeding in relation to hip fracture [with confidence intervals (CIs) that included the null value]; however, that association could not be reproduced when the comparison was limited to parous women. In contrast, a negative association was reported by Cumming and Klineberg that persisted when the comparison was restricted to parous women; however, CIs for the measure of association included the null value. A study conducted in southern France showed no association of breastfeeding with subsequent fracture .
The most compelling evidence for a protective effect of lactation on subsequent fracture risk was reported by Bjørnerem et al. who studied 4681 postmenopausal Norwegian women over 15 years for hip, wrist, and proximal humerus fractures and noted no overall difference in fracture rates between parous and nulliparous women. However, women who breastfed had a 50% lower risk of a hip fracture and 27% lower risk of any fracture, and a marginally significant trend for a negative association of lactation duration and hip fracture risk after adjusting for BMI and relevant covariates.
23.5.4
Studies with bone turnover markers
Evidence supporting the observation of acute bone mineral loss and subsequent remineralization also comes from cross-sectional and longitudinal measurement of bone turnover markers and markers of calcium homeostasis Concentrations of osteocalcin and bone-specific alkaline phosphatase reached their zenith in the early postpartum period and subsequently declined. Holmberg-Marttila et al. found that both markers of formation and resorption were elevated at parturition and remained so in the early postpartum period but extended the findings by identifying both higher parity and a longer history in the postpartum period compared to previously nulliparous women of the same age. The mechanism that might account for this accommodation has not been described. Carneiro et al. noted comparable changes in bone turnover markers with lactation in African-American women compared to historic Caucasian controls despite lower 25 OH vitamin D and higher PTH, suggesting a consistent mechanism across ethnicities .
23.5.5
Calcium and vitamin D supplementation during pregnancy and lactation
Recommendations for calcium and vitamin D supplementation during pregnancy and lactation have generally not been data driven, but several studies are beginning to document demonstrable results. Diogenes et al. reported higher lumbar spine BMC, lumbar spine BA, and lumbar spine BMD than placebo controls at 20 weeks postpartum in 30 adolescent Brazilian mothers supplemented with daily calcium (600 mg) plus vitamin D3 (200 IU) from 26 weeks of pregnancy until parturition . Ettinger et al. described decreased bone resorption through 1-month postpartum as measured by the effect on urinary cross-linked N-telopeptides (NTx) of type I collagen of a double-blind, randomized placebo-controlled trial of oral calcium 1200 mg/day initiated in the first trimester . However, in a randomized, controlled, double-blind trial of three daily doses of vitamin D (400, 2000, and 4000 IU/day) supplemented between 12–20 gestation and 0–14 weeks postpartum in 301 women, Wei et al. noted no differences by dose in changes in BMD or BMC, whereas African-American ethnicity and obesity were associated with greater loss of spine BMD and femoral neck BMD, respectively . Similarly, a trial of 174 women randomized to receive either 400 or 1200 IU of vitamin D/day while breastfeeding for the first 6 months postpartum noted only a slightly higher serum 25(OH)D level at 6 months but no differences in body composition or bone mass . Further studies of dose, duration, timing of administration, and outcomes of calcium and vitamin D supplementation are necessary to provide solid evidence for future recommendations.
23.5.6
Summary and implications
In summary, there appears to be little ultimate loss of mineral from the maternal skeleton with lactation of well-nourished women, during or after lactation, if menstrual cycling is reestablished. Current evidence indicates that extended lactation is associated with acute skeletal loss despite high dietary calcium intake. Variation in the calcium intake was not related to the amount of bone lost in either well-nourished or poorly nourished women. Likewise, calcium intake was not significantly associated with changes in bone turnover markers. The time to return of menses was consistently associated with time of bone mineral recovery.
Presently, there is much to be learned about the mechanisms associated with the rapid loss during lactation as well as the rapid recovery of bone mineral that follows weaning. Investigations of PTH-rP concentrations have been associated with the bone loss of lactation. Understanding these mechanisms could possibly be extended to other bone loss processes, including those associated with menopause, and potentially could serve as a model for facilitating bone mineral recovery.
23.6
Ovarian activity or menstrual cycle characteristics and bone mass
The endocrinology of the ovarian cycle and the physiological manifestation in the menstrual cycle have not been well studied in relation to bone mass. This section addresses the onset of the menstrual cycle and explores the effects of subclinical and clinical disruption of the ovarian cycle.
23.6.1
Age at menarche
The initiation of menses and accompanying estrogen surge may stimulate bone growth by increasing osteoblastic activity . However, the role of age at menarche relative to BMC could be defined more clearly if we understood whether age at menarche was related primarily to bone growth (and epiphyseal closure) or greater likelihood of mineralization as an adjunct to the increased likelihood of greater body size, or equally to both. In addition, defining the initiation event for menarche, that is, hormone sensitivity or critical body fat mass, would also allow greater understanding about the long-term impact of the age at menarche on bone mass.
Those with earlier age at menarche establish ovulatory cycles more quickly than girls with later age at menarche. Likewise, young women with early onset of menarche demonstrated greater concentrations of estradiol and follicle-stimulating hormone (FSH) as compared to young women with later onset of menarche, while maintaining comparable body weights .
Two major hypotheses have emerged to explain the variation in age of menarche: one related to triggering of the pituitary–gonadal axis by maturation and the second associated with the achievement of a critical weight (body fat). Grumbach et al. hypothesized that the onset of puberty is the result of decreasing hypothalamic sensitivity to gonadal steroids. The hypothesis postulates that the decreasing sensitivity results in increased output of the gonadal steroids (positive feedback), which ultimately brings about the morphologic and physiologic characteristics of sexual maturity. If this is the mechanism for menarche, it would imply that women with a delayed onset of menses might fail to establish higher concentrations of the gonadal steroids required for the feedback process. Such adolescents may have lower BMD if there is continued failure throughout early adulthood to establish a “normal” menstrual cycle pattern.
The early version of the critical weight (body fat) hypothesis elaborated by Frisch and Revelle proposed that menarche is achieved by attaining a critical body mass (as reflected by total weight). A secondary data analysis of three longitudinal growth studies suggested that the critical weight was 47.8 kg. The hypothesis was subsequently revised to suggest that the essential component of the body mass was in the fat compartment and that the critical fat level was 17% . Frisch linked the critical fat hypothesis to hormone levels through the work of Nimrod and Ryan , who developed the concept of aromatization of androgens in body fat as sources of the estrogen estrone. The hypothesis has been highly criticized for its methodological and empirical limitations (reviewed by Scott and Johnston ). Whether weight acts as the precipitating or secondary event in the initiation of menarche, low weight (as a mechanical force) and low body fat mass (that becomes compromised as a secondary source of estrogens by the aromatization of androgens) have been suggested as risk factors for lower peak bone mass.
A study of more than 2600 women in 512 pedigrees acknowledged the importance of environmental factors in age at menarche but suggested that the association was primarily attributable to shared genetic contributions rather than environmental factors . This does not preclude the body fat hypothesis but may change the orientation to the genetic component of body fat accrual.
23.6.2
Studies of bone mineralization and age at menarche
Numerous studies suggest that age at menarche is associated with bone growth and bone density. It has been observed that girls with an earlier onset of menarche are shorter, heavier, and have a shorter duration of bone growth than girls of usual age at menarche . Conversely, girls with late age at menarche (14 years) are more likely to be taller and have lower body fat and lower bone density . Later age at menarche is a risk factor for lower BMD , lower trabecular bone score , and for more rapid rate of premenopausal bone loss . In the later study, there was no relation between age at menarche and radial BMD when nulliparous women were removed from analysis. Possibly, the hormonal environment that is associated with failure to conceive is the same environment associated with delayed puberty .
Age at menarche can be related to bone mineralization in at least two different ways. First, women with an earlier age at menarche are likely to have a longer time between menarche and menopause (gynecological age), a time during which estradiol resources are available to support and maintain bone mineralization. Second, events that precipitate earlier menarche, including weight gain, may be associated with characteristics that have been reported to produce greater bone density and, by imputation, greater peak bone density. Emerging evidence suggests that some genes regulating skeletal features of bone geometry are correlated with age at menarche .
23.6.3
Menstruation and number of menstrual cycles
One reason that disparities may exist in assessing the role of reproductive factors is that the various events markedly alter the likelihood of exposure to specific levels of hormones. For example, with pregnancy and lactation, the effect of the elevated estrogen levels of pregnancy followed by the suppressed levels during lactation may generate a cumulative influence on bone density quite different from the influence of each event alone. One approach to accommodate these normal fluctuations in hormone levels is to examine the number of menstrual cycles. Fox et al. showed a positive association between radial bone density in postmenopausal women with each successive year of continued menstruation. Georgiou et al. reported that BMC in postmenopausal women was better explained by the total number of menstrual cycles than by the years since menopause or chronological age. Two reports indicate that women who always had irregular cycles had an increased risk of hip fractures compared to those who never had irregular cycles as did women with infertility .
23.7
Dysfunctional ovulation
23.7.1
Marginal hormone status
Although the prevalence of frank estrogen deficiency has been estimated to be approximately 2% in college-aged women, the prevalence in a general population, ages 20–40 years, is not well established. Furthermore, subclinical levels of estrogen insufficiency may be more common and may influence bone density. Several studies have suggested that marginal hormone status is important in establishing variation in premenopausal BMD.
Marginal hormone status associated with low premenopausal bone mass has been reported in three studies. Sowers et al. described a nested case–control study in which significantly lower estradiol and testosterone concentrations and higher LH values were found in the low BMD group than in the control group. In a subsequent study, Sowers et al. showed that daily urinary hormone excretion patterns for women with lower peak BMD differed from those of women with normal BMD. Healthy, menstruating women with low BMD from a large population-based study had significantly lower urinary sex steroid hormone concentrations during the luteal phase of menstrual cycles compared to hormone concentrations in premenopausal women with average BMD, even after considering the role of body size. Notably, LH peaks were lower and there was a muted progesterone response. These data suggest that subclinical decreases in circulating gonadal steroids may impair the attainment and/or maintenance of bone mass in otherwise reproductively normal women. Steinberg et al. reported lower serum estradiol concentrations in perimenopausal women (mean age of 46 years) versus premenopausal women (mean age of 41 years). Free estrogen and free testosterone concentrations were positively correlated with bone density. These hormone characteristics were observed in populations without anorexia nervosa or intense chronic physical activity. In the SWAN, Grewal et al. reported that lower urine estrone conjugates and higher urine FSH were associated with lower spine and hip BMD in premenopausal and early perimenopausal women, but that short luteal phases or anovulatory cycles were not associated. These associations were consistent across four ethnic groups.
23.7.2
Subclinical ovulatory disturbances
Some women with regular menstrual cycles experience subclinical ovulatory disturbances (cycles that are anovulatory or with a short luteal phase). Hormonally, these cycles are characterized by lower levels of progesterone but normal levels of estradiol. Progesterone may play a central role in bone formation and maturation through osteoblast receptors, but it does not appear to impact bone resorption . Thus women with chronically low levels of progesterone across menstrual cycles may not have adequate bone formation to counterbalance the small but regular increases in bone resorption that occur with estradiol declines during the luteal phase of the menstural cycle . A metaanalysis of six prospective, observational studies and a combined sample size of 436 women found that premenopausal women with more frequent ovulatory disturbance but regular menstruation had greater loss of spinal BMD .
23.7.3
Pronounced events of ovarian dysfunction
Two syndromes that include amenorrhea, chronic endurance exercise and anorexia nervosa, have been characterized relative to bone density. It has been assumed that amenorrhea in both of these syndromes arises from reductions in total body fat rather than from intrinsic disruption of the neuroendocrine system. Two other clinical entities, prolactin-secreting tumors and polycystic ovarian disease, are less extensively studied relative to bone mass and are assumed to have primary involvement of the neuroendocrine system.
23.7.4
Chronic endurance exercise
Premenopausal athletes are typically characterized by low body fat, less body mass, and greater BMD than nonathletes. However, it has long been appreciated that pre- and perimenopausal women who engage in chronic endurance exercise, if accompanied by menstrual dysfunction, may be catabolic rather than anabolic for bone . The impact of long-distance training on female high school athletes is difficult to differentiate from osteopenia of adolescence as they achieve peak growth velocity, particularly in cross-sectional studies. For example, Kaga et al. reported higher levels of osteocalcin and tartrate-resistant acid phosphatase and lower levels of BMD in high school athletes compared to adult athletes, although a number of investigators have identified a peak in height velocity and bone metabolite circulation around age 16 years . Kaga et al. concluded that the effect of long-distance training was different in adolescent versus adult athletes but did not account for the differences that might be observed during adolescence by including a control group of adolescents who did not engage in long-distance training.
Reported menstrual cycle changes in women who exercise strenuously include delay in menarche , shortened luteal phase of the menstrual cycle , menstrual irregularities, oligomenorrhea, and amenorrhea . Some investigators have suggested that the HPO–adrenal axis is suppressed by rigorous physical activity; subsequently, bone mass is lower because of lower concentrations of estradiol and progesterone and higher concentrations of cortisol . Another hypothesis is that lower BMD in female athletes is the consequence of repeated episodes of hyperprolactinemia , although increased basal prolactin values have not been identified consistently in amenorrheic athletes . Other studies have shown that progesterone, prolactin, and testosterone concentrations all increase with strenuous physical activity .
Frisch argued that amenorrhea of exercise is due to a diminution of critical weight (fat mass). She further argued that there is a state associated with transitory weight recovery or moderate physical training that is accompanied by menstrual cycles that occur with shortened luteal phases or that are anovulatory. The critical weight hypothesis is not well supported in the literature, which indicates that both eumenorrheic and amenorrheic athletes may have similar amounts of body fat. For example, Myburgh et al. found that amenorrheic athletes had lower BMD than controls, matched on age, body mass, and exercise quantity. This lower BMD was observed at the spine, proximal femur, and total body, but not at the midradial or tibial shafts. Linnell et al. suggested that discrepancies observed in describing relationships between intense physical exercise and BMD may reflect the additive effect of low body fat and intrinsic ovarian dysfunction, indicating that these are not consistently simultaneous events. Prior et al. concluded that decreases in spinal BMD among eumenorrheic women athletes correlated with asymptomatic disturbances of the ovulatory cycle and not with the degree of physical activity. Physical stress alone can influence menstrual cycling, regardless of body fat levels. Barrack et al. noted that, in runners aged 13–18 years, menstrual irregularities, participation in five or more seasons of endurance running, BMI, and lean tissue mass were independent predictors of low BMD.
Female athletes who engage in high energy expenditure exercise coupled with inadequate energy intake experience a condition known as low energy availability, which may result in menstrual disturbances, thereby leading to detrimental effects on BMD and bone microarchitecture . This collection of outcomes—low energy availability, menstrual disturbances, and low BMD—is known as the female athlete triad . Women with a higher number of risk factors for the female athlete triad have increased risk of low BMD and bone stress injuries . However, even among eumenorrheic women, induction of low energy availability through diet induces reductions in P1NP, a marker of bone formation .
While the catabolic effect of amenorrhea and strenuous endurance sports on bone mass in women is relatively consistently observed, demonstrating anabolic effects of fitness and moderate physical activity is more problematic. Potentially, fitness and moderate physical activity could be anabolic for bone by either hormonal mechanisms or increasing the mechanical loading on bone. This effect, however, may be dependent upon the menarcheal status; female athletes participating in high-impact sports had higher BMD than those participating in low-impact sports, and this effect was most pronounced after menarche . Another consideration of the relative impact of reproductive function and mechanical loading on bone is the type of bone in question. In a cross-sectional study of young exercising women by Mallinson et al., amenorrheic women had lower lumbar spine BMD as compared to ovulatory women, but differences in BMD at the hip was more strongly associated with lean mass than with reproductive function . A hormonal effect associated with physical fitness and body composition may be mediated through an increase in the secretion of growth hormone and thus somatomedin-C or IGF-I. This hormone apparently stimulates the intermittent secretion of PTH, collagen synthesis, and number of osteoblasts . The effect may also be mediated through alterations in leptin, which is positively correlated with fat mass, and can impact bone metabolism through both central and peripheral pathways .
23.7.5
Amenorrhea of anorexia nervosa
Osteoporosis is an established complication of anorexia nervosa and the condition is associated with greater risk of adolescent and early-adulthood fractures (see also Chapter 17: Epidemiologic methods in studies of osteoporosis ). Proposed mechanisms for osteopenia include estrogen deficiency, glucocorticoid excess , generalized malnutrition, calcium intake deficiency, acquired growth hormone resistance, and dysregulation of hormones with anabolic effects, including leptin, insulin, and amylin , with the potential for more than one mechanism to be operating simultaneously. One hypothesis links decreased serotonergic signaling with activation of the sympathetic nervous system inducing decreases in bone mass through β2-adrenergic signaling in osteoblasts . While some investigators have reported that the compulsive exercise frequently associated with anorexia nervosa was protective for bone loss , others have failed to observe this protective relation . This discrepancy may be related to the degree and intensity of exercise practiced by study participants. In a Danish registry of persons diagnosed with anorexia and bulimia, fracture risk was almost twofold greater (risk ratio 1.98, 95% CI 1.60–2.44) in cases as compared with controls .
Because women with anorexia nervosa are frequently both underweight and amenorrheic, ascertaining the independent contributions of estrogen deficiency and decreased body mass to their osteopenia is difficult. However, in hyperprolactinemic amenorrhea, women with increased body weight are protected against osteopenia. This suggests the potential for independent contributions from both the underweight and hypoestrogenism . Bachrach et al. found body size, age at onset, and duration of anorexia nervosa, but not dietary calcium intake, physical activity level, or duration of amenorrhea to be correlated with BMD in adolescent girls. Dietary calcium supplementation has not promoted bone mineral maintenance; however, most studies acknowledge concerns about patient compliance with the therapy and short duration of therapy . With rare exception , studies have failed to differentiate whether the subjects were women who had failed to acquire bone or women who had lost bone.
23.7.6
Hyperprolactinemia
Gonadal suppression with prolactin-secreting tumors and other conditions associated with hyperprolactinemia may be an important contributor to low premenopausal bone mass and subsequent risk of osteoporosis in a limited number of women. Prolactin may exert direct effects on bone remodeling through decreased osteoblasts and increased activity of bone resorption through the RANKL/OPG pathway . The incidence of hyperprolactinemia peaks during the third to fourth decade, and its prevalence is estimated to exceed 25% among young adult women with amenorrhea.
In a longitudinal study by Schlechte et al. , women with hyperprolactinemia had lower bone mass of the spine and radius at entry to the study. Over the 4.7-year follow-up, women with hyperprolactinemia did not lose bone mass, whereas healthy women had significant loss at the spine (but not radius). The investigators suggest that women with hyperprolactinemia may have retained bone mass in the face of decreased estradiol concentrations because of greater body mass (28 vs 24 kg/m 2 ) and higher testosterone concentrations. Restoration of gonadal function was not associated with normalization of the bone mineral .
Klibanski and Greenspan also reported that treatment improves bone density in women with hyperprolactinemia but does not return bone density to the level observed in controls. However, it has been observed that hyperprolactinemic women who were eumenorrheic had greater bone density than hyperprolactinemic women who were amenorrheic , suggesting the potential for a differential response according to the duration of reduced estrogen stimulation.
Importantly, while hyperprolactinemia has been associated with decreased bone mass, weight gain, insulin resistance, and endothelial dysfunction, it has not been associated with increased fracture rates .
23.7.7
Polycystic ovarian syndrome
Polycystic ovarian syndrome (PCOS) is a heterogeneous group of conditions characterized by polyfollicular ovaries and an LH-dependent increase in androgen secretion. In addition to oligomenorrhea, this multifaceted syndrome may be accompanied by various degrees of virilization, obesity, hypertension, and diabetes. Di Carlo et al. compared 188 women diagnosed with PCOS to a similar group of 142 patients with normal ovaries and reported that women with PCOS had significantly greater bone density of the lumbar spine (0.98 vs 0.87 g/cm 2 ). The same group also reported higher serum concentrations of LH, prolactin, and, as expected, testosterone. The investigators speculated that several factors may be associated with the greater BMD in the face of amenorrhea in this group. The PCOS group had a greater BMI (25.0 vs 22.9 kg/m 2 ) than women with normal ovaries and had higher androgen levels. More recently, using peripheral quantitative computed tomography, Kassanos et al. found higher tibial volumetric cortical density in both obese and lean women with PCOS than control women, suggesting that mechanical load is insufficient to explain higher BMD . Other studies, however, have found that women with PCOS have lower or no difference in BMD than women without PCOS. Katulski et al. found that PCOS women of normal body weight had lower BMD than did non-PCOS women of normal body weight, thereby suggesting that previous findings of PCOS and bone density may be confounded by body weight. In a case–control study where PCOS cases were matched to women without PCOS by age and body weight, Ganie et al. found no difference between the BMD of case and control women .
23.7.8
Summary and implications
Among premenopausal women, there are variations in ovarian and gonadotropin hormones associated with variation in BMD. While the frank amenorrhea that may accompany chronic endurance physical activity, hyperprolactinemia, PCOS, and anorexia nervosa has long been recognized as being associated with lower BMD, the prevalence of these conditions is uncertain. Thus it is difficult to ascertain the overall impact on peak bone mass and, by extension, osteoporosis, and fracture. New studies now indicate that lower concentrations of hormones jeopardize BMD even when amenorrhea is not present. This suggests that, as more is learned about the relationship between peak bone mass and osteoporosis risk, premenopausal hormone administration may become a more prominent source of intervention.
23.8
Oral contraceptive use
The impact of OCPs on BMC has been of great interest as investigators have tried to determine parallels between OCPs and menopausal hormone therapy on bone density. The lines between OCPs and estrogen therapy use have become increasingly blurred. OCPs are now approved for use in women over the age of 35 years, and estrogens are the common constituent frequently associated with both OCP products and menopausal hormone therapy. However, there are major differences in the drug formulations, including the presence or absence and types of progestins, the dosage of active ingredients, and the regimens for their use. The impact of OCP use on BMD remains unresolved, with studies reporting both no effect and a positive effect.
In this review, both cross-sectional and longitudinal studies of OCP use and BMC were examined and, when possible, dichotomized according to menopausal status. This dichotomy is useful for the following reasons. First, formulations for OCPs used by women prior to 1980 (who are now more likely to be peri- and postmenopausal) generally had significantly higher estrogen doses than the preparations to which most premenopausal women have been exposed. Second, there may have been different selection factors operating as to which women elected to use OCPs in the 1960–70s versus those currently using OCPs.
23.8.1
Studies of oral contraceptive use and bone mass
Findings from studies of OCPs and bone mass have been inconsistent, despite the substantial number of reports. A report from the Cochrane Library reviewing findings from randomized controlled trials concluded that combined oral contraceptives were not detrimental to BMD but that the formulations of the hormonal contraceptives varied across studies . Thus whether OCP preparations are associated with greater BMD remains debatable. Numerous cross-sectional studies have reported a positive association between bone density and OCP use in various populations of premenopausal women. Likewise, several studies have reported a positive association of OCP use across a wide age range, including postmenopausal women . In contrast, other cross-sectional studies reported no association of OCPs with BMD in premenopausal women and among women across a wide age range .
Analyses of findings based upon longitudinal studies are invaluable to enhancing our understanding of the association between OCP use and bone density as the relationship appears to depend upon bone site, and duration and timing of use. In one of the few longitudinal studies, Recker et al. reported a positive correlation between total body bone mineral and OCP use; however, they observed no association between OCP use and BMD of the forearm or lumbar spine. In a longitudinal study with 5 years of observation, Sowers et al. reported that among 22 pre- and perimenopausal women who had ever taken OCPs for more than 3 months, a longer duration of use was associated with less radial BMD loss, after adjusting for age. In a study following women from adolescence into young adulthood, Jackowski et al. reported that the timing of exposure to combined hormonal contraceptives was critical to the association with BMC and BMD. Participants who used combined hormonal contraceptives during adolescence (1 year after attainment of peak linear growth) have slightly higher total body and lumbar spine BMC as compared to those without combined hormonal contraceptive use. However, use of the contraceptives 6+ years after attainment of peak linear growth was detrimental to total BMD .
It appears that an underlying assumption of BMD studies is that OCP preparations increase the circulating estrogen concentrations. However, many of the current preparations provide hormone doses just adequate to suppress ovulation and not sufficient to generate the variation in physiologic ranges found throughout the menstrual cycle in women not using OCPs. Most studies evaluating the potential effect of OCPs on BMD reflect ethinyl estradiol dosages of 35 μg or greater. A study evaluating low-dose (20 μg) OCPs found BMD reduced in women using that pill . There is good reason to believe that OCPs may help promote bone mineralization in women with very low circulating hormones, amenorrhea, or oligomenorrhea. There is less likelihood that BMD will be retained if the use of OCPs actually lowers circulating estrogen concentrations in any particular woman. Indeed, a study by Garnero et al. indicated that there was no overall difference in BMD between users and nonusers; however, OCP use was associated with a moderate decrease in bone turnover.
23.8.2
Studies of oral contraceptives and fractures
The number of studies of OCPs and fractures is limited, in part because women who were of an age to use OCPs in the 1960s and 1970s are now achieving an age where fractures occur with sufficient frequency to make such a study efficient. A Swedish case–control study of OCP use and fractures suggested that the use of OCPs in late reproductive life may reduce hip fracture risk in postmenopausal women (odds ratio 0.75, 95% CI 0.59–0.96) , which is in contrast with the experience in the 46,000 enrollees in the Royal College of General Practitioners Oral Contraception Study. The risk of subsequent fractures was significantly greater among OCP users than among nonusers . In the Women’s Health Initiative Observational Study, there was an adjusted relative hazard for fractures among past OCP users of 1.07 (95% CI 1.01–1.15), suggesting a very modest increased likelihood of having fracture in a cohort of 93,725 women aged 50–79 years . A Cochrane review found no randomized clinical trials of hormonal contraceptives with fractures as a clinical endpoint , not surprising given the usual ages of participants in such trials and the long lag before reaching significant fracture rates.
23.8.3
Summary and implications
While there have been a substantial number of studies that relate bone mass and OCP use, ambiguity remains. Findings about OCPs and bone mass may be difficult to synthesize for the following reasons. First, only some of the progestrogens are 19-nor-testosterone derivatives that have androgenic/anabolic properties. For example, Cundy et al. reported that the degree of estrogen deficiency induced in women using depot medroxyprogesterone acetate (DMPA) for contraception may adversely affect bone density (see later). This is evidence for the importance of formulations of the particular OCP. Second, dose and duration of use may have a differential impact according to the chronological or gynecological age of the user. For example, the role of menopause may overshadow any impact of OCP use on BMD in postmenopausal women. The OCP effect may be different in adolescents still acquiring bone as compared to adult women who are more likely to be in a bone maintenance phase. Third, OCPs are also used in the regulation of dysfunctional menstrual cycles. As such, the universe of OCP users may be quite heterogeneous and include women with conditions that include potential hormonal abnormalities, for example, dysmenorrhea or irregular cycles, as well as women who use the hormones for contraception alone.
Any future studies of OCP use should be undertaken in women in whom it can be determined if the hormonal preparation is being used for conception prevention or menstrual cycle regulation. Duration of use, as well as dose and type of the preparation, should also be addressed. In younger women the issues of OCP use in bone acquisition versus bone maintenance should be addressed. In older women the potential bone loss with age and menopausal status should be separated from the impact of duration of OCP use.
23.9
Progestin-injectable contraceptives
A progestin-only, injectable contraception, DMPA, given intramuscularly every 90 days, was approved for use in the United States in 1992. Worldwide, the contraceptive DMPA is used in more than 90 countries by an estimated 3.5 million women. There is a compelling physiological mechanism by which DMPA could compromise BMD. Contraception is achieved primarily through disruption of the HPO axis. Because DMPA disrupts the HPO axis, it will suppress estrogen production, leading to a relative estrogen deficiency and a corresponding loss of BMD. If DMPA has an adverse effect on BMD, then a substantial cohort of young women may enter menopause with less bone mineral reserve and be at increased risk for the development of osteoporosis, fracture, and related morbidity following menopause.
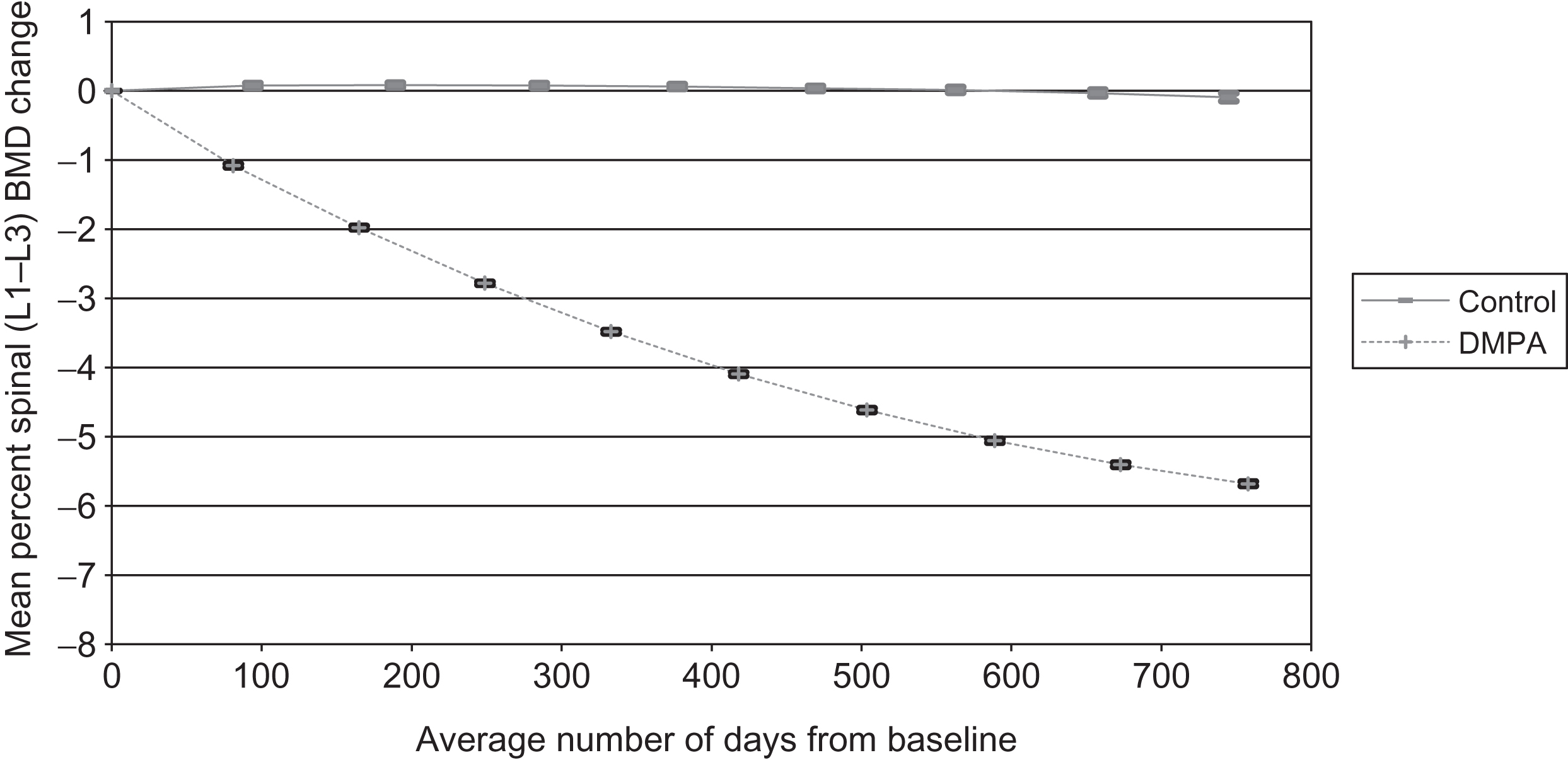
Many studies have addressed potential BMD levels among users. Collectively, they suggest that BMD values were approximately 3%–7% lower than values in controls . A 2-year study of 178 first-time nonadolescent users had mean hip and spine BMD losses of 5.7%, while 145 controls had less than 0.9% loss over the same 24-month period (see Figs. 23.1 and 23.2 ). Increasing BMI among DMPA users offered protection against DMPA-related BMD loss; however, calcium intake, physical activity, and smoking did not influence BMD change in either group . Two other studies of new DMPA users with a smaller number of users reported hip and spine BMD losses ranging from 1.5% to 3.3% . Three longitudinal studies of predominantly long-term DMPA users reported minimal or no BMD loss , but these data did not indicate the status of BMD prior to DMPA initiation. Studies using biochemical markers (osteocalcin and N-telopeptides) indicated that both markers were higher, on average, than oral conceptive users but were not markedly higher than nonusers . Other studies suggest this BMD compromise may resolve following the discontinuance of DMPA use . Clark et al. demonstrated that BMD loss slowed after 48 months of continuous DMPA use and increased after discontinuation from 0.3% to 2.0% per year depending on length of DMPA use and bone site . Harel et al. reported that mean lumbar spine BMD returned to baseline within 60 weeks of the final DMPA injection and increased 4.7% above baseline by 240 weeks . Mean total hip and femoral neck BMD recovered to baseline more slowly, requiring 240 and 180 weeks, respectively, after the final DMPA administration.