Red blood cell (RBC) transfusion is a cornerstone of the management of patients with hematology/oncology disorders. However, a potentially deleterious consequence of transfusion is the development of alloantibodies against blood group antigens present on RBCs. Such alloantibodies can be an obstacle in providing compatible units for transfusion. Providers in this arena must fully understand the testing performed by blood banks, as well as the consequences of detected antibodies. This article reviews immunohematologic tests, describes how autoimmune hemolytic anemia is classified by autoantibodies; outlines RBC alloimmunization rates, and presents strategies to prevent/mitigate the impact of RBC alloimmunization.
Key points
- •
Many immunohematologic tests performed by blood banks, including antibody screening, direct antiglobulin tests, eluates, and minor antigen phenotyping, are relevant to hematology/oncology patients.
- •
Cold and warm autoantibodies may mediate intravascular or extravascular autoimmune hemolysis in hematology/oncology patients.
- •
Transfused individuals with hematologic/oncologic disorders may develop red blood cell alloantibodies, which can complicate pretransfusion testing, delay blood product availability, and lead to transfusion reactions.
- •
Several strategies exist to prevent the development of blood group alloantibodies in transfused hematology/oncology groups.
Introduction
Among the aspects of care rendered to patients with hematologic/oncologic disorders, blood transfusion is common. However, the tests that form the basis for transfusion compatibility and antibody identification are not always well understood, nor are their interpretations always straightforward. This article:
- 1.
Reviews immunohematologic tests performed by transfusion services
- 2.
Describes how autoimmune hemolytic anemias (AIHA) are characterized by autoantibodies detected
- 3.
Outlines red blood cell (RBC) alloimmunization rates described in various hematologic/oncologic patient populations and the potential ramifications of these alloantibodies
- 4.
Presents potential strategies to mitigate RBC alloimmunization
Introduction
Among the aspects of care rendered to patients with hematologic/oncologic disorders, blood transfusion is common. However, the tests that form the basis for transfusion compatibility and antibody identification are not always well understood, nor are their interpretations always straightforward. This article:
- 1.
Reviews immunohematologic tests performed by transfusion services
- 2.
Describes how autoimmune hemolytic anemias (AIHA) are characterized by autoantibodies detected
- 3.
Outlines red blood cell (RBC) alloimmunization rates described in various hematologic/oncologic patient populations and the potential ramifications of these alloantibodies
- 4.
Presents potential strategies to mitigate RBC alloimmunization
Basic tests of compatibility in the blood bank
For hematology/oncology patients, one of the most commonly performed tests is the type and screen. In this testing, an individual’s ABO and Rh(D) status is determined (the type) and the presence/absence of autoantibodies or alloantibodies is established (the screen).
Principle of Hemagglutination and Testing Platforms in the Blood Bank
Of the tests to be discussed later, a basic tenet inherent to all is mixing patient specimen (plasma or RBCs) with a reagent to examine for a reaction. Positive testing in the blood bank setting typically is reflected as hemagglutination. The strength of the agglutination reaction is graded on a 0 to 4+ scale, with 0 indicating a negative test (ie, no agglutination) and 4+ indicating an overwhelmingly positive reaction.
Hemagglutination can take place via several laboratory platforms. Historically, compatibility testing was performed in glass or plastic tubes, with patient specimen and reagent added, and, after several incubation and wash steps, observation for agglutination. Given the moderate sensitivity and laborious nature of the tube method, several technological advancements were subsequently introduced. Although still based on the fundamental principle of agglutination, platforms such as automated gel columns have been developed to further augment a blood bank’s ability to detect RBC antibodies. More recently, automated platforms involving solid phase RBC adherence (wherein a patient’s plasma is mixed with immobilized RBC antigens on a plate or well) have been accepted into clinical practice. Although gel and solid phase platforms have increased sensitivity compared with tube methods, this comes with a concomitant increase in false-positive reactions.
ABO and Rh(D) Blood Group Phenotyping
The ABO system, representing a series of carbohydrate antigens on the RBC surface, is the single most important blood group family for transfusion compatibility. Infusion of ABO incompatible blood components can result in severe hemolytic reactions, morbidity, and death. ABO antigens are particularly problematic because individuals lacking a given antigen within this system make a corresponding antibody from early in life without the need for prior RBC exposure via transfusion or pregnancy. As such, thorough and reliable mechanisms for determining an individual’s ABO status are critical for compatibility.
Blood banks typically perform 2 steps in determination of an individual’s ABO status: forward grouping for the presence or absence of A or B antigens (informally referred to as the front type) and reverse grouping for the presence of anti-A or anti-B antibodies in plasma (informally referred to as the back type). The front type involves detection of A or B antigen using reagent antibodies directed against these targets. The back type is meant to serve as a confirmation of the results of the front type and is based on the previously described principle that an individual lacking a given ABO antigen should have a detectable antibody against that antigen.
In terms of clinical significance, the Rh blood group antigen system is the second most important after ABO. Unlike ABO antigens, which are carbohydrate in nature, Rh antigens are polypeptide and typically require a previous exposure before induction of an alloantibody. Also unlike the ABO system, the Rh system is highly complex with more than 30 unique antigens. However, during routine practice, blood banks only test for the presence or absence of a single antigen in the Rh system: Rh(D).
Phenotyping for the other, non-Rh(D), minor blood group antigens may also be completed for select patient populations. Such phenotyping for antigens including C/c, E/e, and K is also typically done by mixing patient RBCs with antisera against these antigens and can be completed with tube or automated methods. As described in more detail later, determining an extended antigen phenotype is typically reserved for patients at high risk for RBC alloimmunization.
Complex tests of compatibility and/or antibody identification in the blood bank
Besides the basic tests of compatibility described earlier, blood banks are often called on to perform more additional tests to identify autoantibodies and alloantibodies (described later and in Table 1 ).
| IAT a | RBC Panel | Antibody Identified in RBC Panel | DAT a IgG | C3 | RBC Elution | Antibody Identified in RBC Elution | Cold Screen | DL Test | |
|---|---|---|---|---|---|---|---|---|
| Alloimmune Processes | ||||||||
| Primary immune response to pregnancy or transfusion | + | + | Specific IgG alloantibody | −/− | −/− | − | None | − | − |
| Anamnestic response or delayed hemolytic transfusion reaction | + | + | Specific IgG alloantibody | −/+ | −/+ | + | Specific IgG alloantibody | − | − |
| Autoimmune Processes | ||||||||
| Warm autoimmune hemolytic anemia | +/− | +/− | Panagglutinin | + | −/+ | + | Panagglutinin | − | − |
| Cold agglutinin disease | − | − | None | − | + | − | None | + | − |
| Mixed autoimmune hemolytic anemia | + | + | Panagglutinin | + | + | + | Panagglutinin | + | − |
| Paroxysmal cold hemoglobinuria | − | − | None | − | + | − | None | − | + |
a For results indicating ‘+/−‘ or ‘−/+’, testing in this setting could give rise to either negative or positive results, depending on the clinical scenario and the antibodies involved. For DATs, expected results of IgG testing are the top line result, whereas C3 results are on the bottom line.
Indirect Antiglobulin Test
The type and screen is the most frequently performed test in the blood bank. When providers order a screen, blood banks are performing an indirect antiglobulin test (IAT) to identify possible autoantibodies or alloantibodies in patients’ plasma. Fig. 1 provides an overview of the IAT, which involves mixture of patient plasma with screening cells expressing various combinations of clinically relevant, non-ABO antigens. IATs are designed primarily to detect immunoglobulin (Ig) G class antibodies. If an IAT is negative, then there is no evidence for an existing autoantibody or alloantibody in the patient’s plasma and blood banks will provide RBCs for transfusion. However, if an IAT is positive, blood banks must identify the cause of that positive reaction such that compatible units for RBC transfusion can be provided. As such, blood banks reflex positive IATs to RBC antibody panel testing.
Identification of Red Blood Cell Antibodies by Panel Testing
The panel assay is performed using a mixture of patient plasma and numerous reagent cell lines. In many ways, panel testing is analogous to the simple IAT screen, with the most significant difference being the number of reagent cells used (10–12 in the panel vs 2–4 in the screen). Selection of a larger number of reagent cells in the panel helps blood banks more clearly identify the antibodies driving the positive reaction.
Note that not every positive IAT reaction is attributable to an alloantibody. Warm-reactive autoantibodies frequently give rise to positive screening tests. However, compared with the results seen with an alloantibody, panels for patients with autoantibodies generally show panagglutination; that is, reactivity with every reagent cell tested. Such a finding typically warrants the performance of a direct antiglobulin test (DAT).
Direct Antiglobulin Test
The DAT is a highly sensitive assay used to detect antibody or complement bound to the RBC surface. As shown in Fig. 2 , DATs are typically 2-step tests, with the first step involving a polyspecific reagent detecting either IgG or complement component C3 on the RBC surface. Agglutination with polyspecific reagents requires additional testing to determine whether IgG, C3, or both are on the RBC surface.
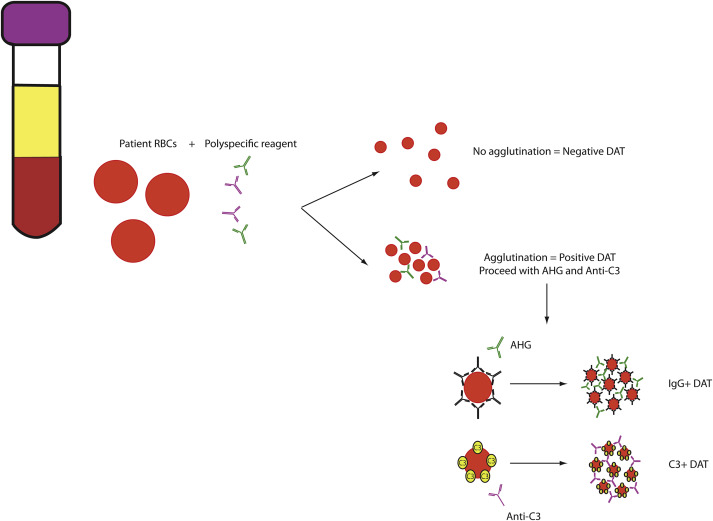
A positive DAT with IgG or C3 may be suggestive of a hemolytic picture, with IgG reactivity often correlating with a warm-reactive antibody/extravascular hemolysis and C3 reactivity typically correlating with a cold-reactive antibody/intravascular hemolysis. However, as a highly sensitive test, the DAT can also be prone to false-positive reactions. Causes of false-positive DATs (ie, agglutination in the absence of immune-mediated hemolysis) include the following:
- •
Administration of drugs that modify the RBC surface (eg, antibiotics)
- •
Polyclonal hypergammaglobulinemia
- •
Previous transfusions
- •
Severe RBC rouleaux
Red Blood Cell Elution
RBC elution is used to evaluate the specificity of an antibody bound to the RBC surface. Although several laboratory techniques are available to help liberate antibodies from RBCs, many blood banks use an acid elution approach for optimal removal. As outlined in Fig. 3 , the interaction between RBC and antibody is weakened in an acidic environment, allowing formerly bound RBC antibodies to accumulate in the plasma. Specimens are then centrifuged and the supernatant plasma removed. The antibody-concentrated supernatant is then reacted with an RBC panel to identify the antibody specificity.
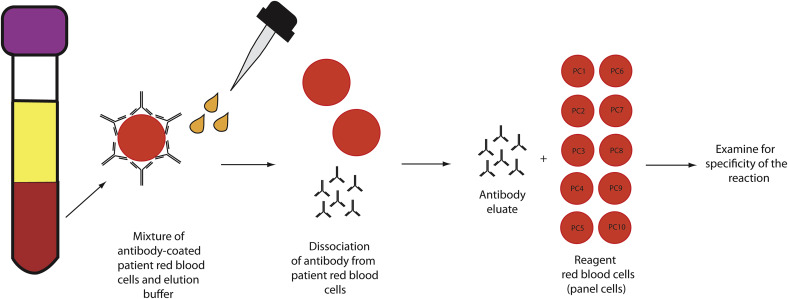
In the setting of AIHA, in which autoantibodies are targeting highly conserved portions of the RBC membrane, RBC elution studies show panagglutination. In contrast, in the setting of alloimmune hemolysis (eg, following an incompatible RBC transfusion), RBC elution studies typically show reactivity for a specific non-ABO antigen. Note that elution studies are most useful for IgG-positive DATs. C3-positive DATs are frequently associated with IgM antibodies; such antibodies are poorly eluted from the RBC surface and, in most blood bank settings, few reagents exist to detect bound IgM.
Cold Autoantibody Screen, Thermal Amplitude, and Titers
Cold autoantibodies are classically IgM and may be difficult to detect by standard blood bank testing. Therefore, cold screens have been developed to help identify the presence/absence of a cold autoantibody as well as possible targets of the autoantibodies on the RBC surface. In a cold autoantibody screen, a patient’s plasma specimen (collected and maintained at 37°C) is subsequently mixed with adult screening cells (harboring the I antigen), cord-blood RBCs (harboring the i antigen), and the patient’s RBCs (to determine whether any reactivity against self is demonstrable); all testing is done at 4°C. In most forms of cold agglutinin disease (CAD) in adults, cold autoantibodies should show strong reactivity against self and RBCs possessing the I antigen. Such reactivity classically has been described in CAD associated with Mycoplasma pneumoniae infections. In contrast, cold autoantibodies showing strong reactivity against self and i-positive RBCs may suggest CAD associated with Epstein-Barr virus infection.
When clinical suspicion exists for CAD, the cold screen is only the first step because many patients can possess clinically insignificant cold autoantibodies. Differentiating a significant from insignificant cold agglutinin requires clinical correlation in addition to examining the thermal amplitude of the autoantibody and its titer. Thermal amplitude studies involve incubating the patient’s plasma with screening cells at temperatures ranging from 0°C to 4°C through 35°C to 37°C. Most clinically significant cold autoantibodies show reactivity (ie, hemagglutination) at temperatures greater than 27°C to 30°C. Regarding titers, several dilutions of patient plasma are mixed with screening cells at 4°C to determine the strength of the autoantibody. Although no formal guidelines exist, reports in the literature suggest that titers of greater than 1:64 to 1:256 are consistent with clinically significant CAD.
Donath-Landsteiner Test
Paroxysmal cold hemoglobinuria (PCH) is a rare cold AIHA. Most often encountered after infection or vaccination in children, and presenting with severe intravascular hemolysis, PCH is caused by an IgG autoantibody (referred to as biphasic hemolysin) targeting the high-incidence P antigen. The DAT is typically positive for complement only, because the cold-reactive IgG autoantibody dissociates from the patient’s RBCs at the warmer temperature at which the DAT is performed. Physiologically, the biphasic nature of PCH is manifested by autoantibodies binding to RBCs at cold temperatures and fixing complement (phase I). Then, as RBCs warm back toward 37°C, fixed complement is within its optimal thermal range and intravascular RBC hemolysis ensues (phase II). The Donath-Landsteiner test thus involves incubating the patient’s plasma with control RBCs at a very cold temperature (typically 0°C –4°C) allowing any cold-reactive IgG autoantibodies to bind and fix complement. This specimen is then incubated at 37°C, allowing fixed complement to lyse the RBCs. When a PCH autoantibody is present, there should be gross hemolysis observed in the plasma supernatant of specimens incubated at 0°C to 4°C followed by 37°C.
Summary for Complex Tests of Compatibility and/or Antibody Identification
The tests described earlier are used in the assessment of hematology/oncology patients who require transfusion care, or in whom an alloimmune or autoimmune process is suspected. A detailed description of the further diagnosis and treatment of AIHA is beyond the scope of this article and the reader is referred to the June 2015 edition of Hematology/Oncology Clinics of North America for in-depth reviews of warm and cold AIHA. Because of these recent reviews of AIHA, the remainder of this article focuses on the alloimmune response to blood group antigens in patients with hematologic/oncologic disorders.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




