Gompertzian growth kinetics
Fig. 8.1
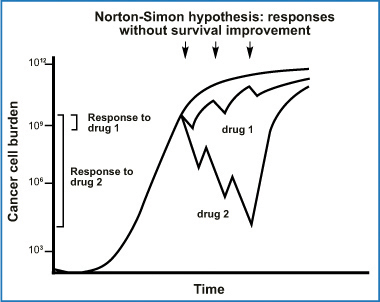
Fig. 8.2
Norton-Simon hypothesis: progressive improvements in drug response do not change actual cancer survival
8.2 Intraperitoneal Chemotherapy: Physical and Biological Principles
The pharmacological rationale behind endoperitoneal chemotherapy consists of dose intensification determined by the peritoneal plasma barrier. The two compartments (peritoneal cavity and blood) are separated by a semipermeable membrane that allows a high peritoneal drug concentration, thus optimizing its effect on the endoperitoneal target and at the same time limiting drug passage into the plasma stream, which causes treatment toxicity. From experience gained in peritoneal dialysis, Dedrick et al. stated that peritoneal permeability to a certain drug is notably lower than the same drug’s plasma clearance. Peritoneal drug clearance is inversely proportional to the square root of its molecular weight [11]. Figure 8.3 shows the equation that describes the pharmacokinetic advantages gained by giving a drug by the endoperitoneal route rather than the intravenous route and the traditional two-compartment model of peritoneal transport with the equation showing the rate of mass transfer. Drugs pass in a parallel fashion from the peritoneal cavity into the various surrounding tissues, and multiplying tissue permeability by the area exposed to peritoneal fluid shows how much a given tissue type contributes to overall transport (Fig. 8.4).
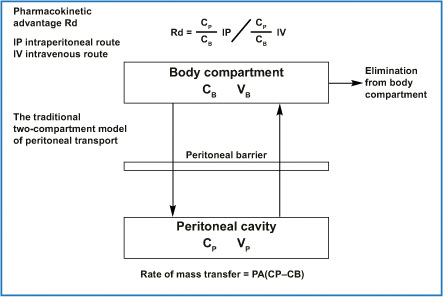
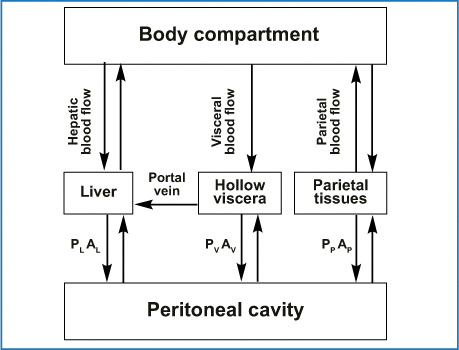

Fig. 8.3
Mass transfer coefficient for a specific drug has conventionally been considered a single parameter. The permeability P could be considered a property of the membrane with units of cm/min; when P is multiplied by the effective area A of the membrane (expressed in cm2), a clearance term, cm3/min, results. It has enabled prediction of peritoneal and plasma concentration and the resulting regional advantage in a clinical setting if the systemic pharmacokinetics are known. PA, permeability area (effective contact area A × permeability of a specific drug P); CP, free drug concentration in peritoneal fluid; CB, free drug concentration in blood; VP, volume of peritoneal cavity; VB, volume of drug distribution in the body

Fig. 8.4
How drug transfer from the peritoneal cavity into surrounding tissue-specific permeabilities P and area A where the subscript can be L for liver, V for hollow viscera, and P for parietal tissue. Low-molecular-weight drugs move from the peritoneal tissues into the rest of the body primarily via blood flow. (Reproduced from [28], with permission)
8.3 Pharmacokinetic and Pharmacodynamic Variables Related to Intraperitoneal Chemotherapy
One reason for delivering chemotherapeutic agents into the peritoneum is that this route allows relatively lengthy contact between the drug and its therapeutic target. The pharmacokinetic advantage of dose intensification arises from the higher drug concentration achieved in the peritoneum than in the plasma, as expressed by the area-under-the curve (AUC) ratio. The simplest way to illustrate the pharmacokinetic advantage is to construct a concentration-time curve. The endoperitoneal AUC reflects therapeutic effectiveness, whereas the intravenous AUC expresses drug toxicity (Fig. 8.5) [12]. A high peritoneal drug concentration does not itself, however, guarantee therapeutic effectiveness insofar as the real pharmacological aim is for the drug to penetrate into the target tumor. An approximate measure of the result obtained is therefore provided by inserting into the preceding graph the chemotherapeutic drug concentration achieved within the malignant nodule (Fig. 8.6) [13]. Hence whereas pharmacokinetic variables (dose, volume of the chemotherapy solution, duration, carrier solution, and pressure) influence peritoneal drug bioavailability; a series of pharmacodynamic variables (size of the malignant nodule, vascularization, interstitial pressure, and temperature) reflect how much drug reaches the oncologic target.
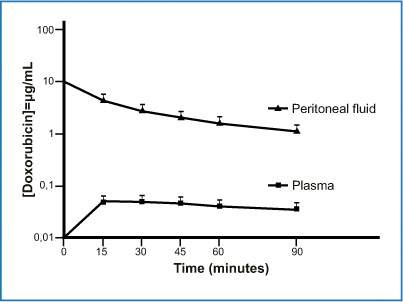
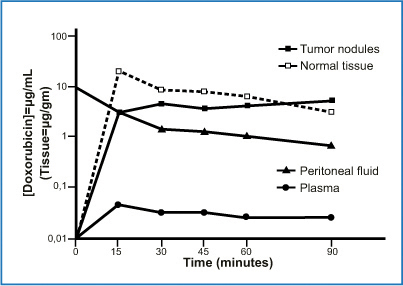

Fig. 8.5
Concentrationtime graph of doxorubicin in peritoneal fluid and plasma in 145 patients. Area under the curve (AUC) ratio of peritoneal fluid to plasma was 78.6 (+ 27.7). (Reproduced from [12], with permission)

Fig. 8.6
Doxorubicin concentration in plasma, peritoneal fluid, tumor nodules, and normal adjacent tissues. Data obtained from a single patient. (Reproduced from [13], with permission)
8.3.1 Pharmacokinetic Variables
A major controversial problem is the wide variability in the chemotherapeutic drug doses used for endoperitoneal chemotherapy. Most groups base the doses delivered, as happens for systemic chemotherapy, on body surface area (BSA) (mg/m2), considering BSA as a peritoneal surface area (PSA). Despite the substantial difference between BSA and PSA [14], BSA is a reasonably reliable method for assessing predictable toxicity. Other groups use a concentrationbased method that calculates the pharmacological dose in mg/m2 per liter perfusate and usually increase this dose up to 6l [15–17]. According to the aforementioned Dedrick formula for transport through the peritoneal membrane, the volume of IP-CHT will increase the solution contact area (A) and will improve mass transfer from the peritoneal cavity to the plasma. The great advantage of a concentration-based system is that the residual tumor nodules after cytoreduction are exposed to increased cytotoxicity, but this advantage pays the price of highly unpredictable systemic side effects. Reported IP-CHT protocols vary in duration from 30 to 120 min. According to a mathematical model proposed by Gardner [18], the dose-response curves and their dependency on exposure time reach a cell-kill plateau, after which prolonging exposure time offers no further cytotoxic advantage. The most advantageous exposure time for cytotoxic effects in peritoneal carcinomatosis (PC) should be weighed against systemic toxicity. Other pharmacokinetic variables include perfusate osmolality (most perfusates are isotonic saline or dextrose solutions) [19] and pressure as a determinant variable reflecting drug penetration into tissues [20]. Hydrostatic pressure, especially for HIPEC, depends partly on the two principal technologies (open vs closed) used, and despite debate over recent years on their advantages and disadvantages, no definitive conclusions have been reached [21]. Two studies conducted by Ortega-Deballon et al. and Facey et al. show in animal experiments (pigs) an increased drug tissue penetration after HIPEC given with the open technique. The investigators obtained the highest tissue concentrations by combining open HIPEC with increased hydrostatic pressure obtained with a special device fixed to the skin margins, and to a Thompson retractor equipped with a vertical latex expander able to increase hydrostatic pressure to 25 cm of water [22, 23].
Pharmacokinetic variables also vary according to the surgical procedure used, and surgical and clinical factors may require changes in chemotherapy administration. In a study conducted on 145 patients with appendiceal cancer and colorectal carcinomatosis who underwent CRS plus HIPEC with doxorubicin as part of a multidrug regimen, Sugarbaker et al. observed that the number of surgical procedures (number of peritonectomy procedures or entity of visceral resections or both) can influence the AUC ratio. Doxorubicin peritoneal clearance differs as the number of peritonectomy procedures or the entity of visceral resections done (gastrectomies or total colectomies) progressively increases (Table 8.1) [12]. The number of peritonectomy procedures increases peritoneal drug clearance, whereas the entity of visceral resections increases the AUC ratio. This observation depends solely on the different rates at which doxorubicin penetrates through the peritoneal-plasma diffusion pathways into the various organs and structures [24].
Table 8.1
Multivariate modeling analysis to evaluate the association between clinicopathologic factors and the area under the curve (AUC) ratio. (Reproduced from [12], with permission)
Clinicopathologic factors | Analysis | P value |
|---|---|---|
Age | Not significant | 0.153 |
Gender | Men have a significantly higher AUC ratio than women | 0.025 |
Completeness of cytoreduction | Not significant | 0.572 |
Peritoneal space | Patients with a restricted peritoneal space have a significantly higher AUC ratio | 0.002 |
Number of peritonectomies | The number of peritonectomies has a significant negative correlation with the AUC ratio (a higher number of peritonectomies tends to have a lower AUC ratio) | 0.003 |
Number of visceral resections | The number of visceral resections has a significant positive correlation with the AUC ratio (a higher number of visceral resections tends to have a higher AUC ratio) | 0.001 |
8.3.2 Pharmacodynamic Variables
Pharmacodynamic variables, expressing what the drug does to the body, focus on the tumor nodule, rightly considering it the pharmacological endpoint for endoperitoneal therapies. The equations in Fig. 8.3 allow us to calculate the pharmacological advantages of endoperitoneal chemotherapy and illustrate drug-transfer mechanisms between the two compartments. However, they provide no information on the real mechanisms that regulate drug penetration into tumor nodules. In vitro experiments with multicellular models show that most cytotoxic agents penetrate poorly into tumor tissue (< 1 mm) [25]. After intraperitoneal cisplatinum administration in an animal model, Los et al., reported a 1- to 2-cm penetration [26, 27]. As the model proposed by Dedrick et al. [28] and the related equation show (Fig. 8.7), low-molecular-weight drugs (up to 6,000 Daltons) present at a given concentration in the peritoneal cavity (Cp) diffuse through the tissues (Ce) according to a concentration that diminishes exponentially until it reaches the concentration in blood (Cb). Drug movement through the tissues is measured by the drug’s diffusion constant (D) (cm2/min) and the rate constant k (min −1), a variable that measures the amount that can be removed from the tissue by blood capillary diffusion. The k constant takes into account capillary permeability, the capillary surface per unit tissue, and capillary blood flow per unit tissue volume. Last, x indicates the distance in centimeters from the serosal surface, assuming that x0 is the distance from the serosal surface at which the concentration difference between tissue and blood has decreased to 37% of its maximum value. The distance from the serosa surface, 3×0, is a reference value at which the concentration difference decreases to 5% of the maximum value. Obtaining increased tissue penetration for low-molecular- weight drugs is no easy task, because it requires an increased D value (diffusion coefficient) or a reduced k value, or both. Drug diffusion into tissue depends on tissue structure and drug properties. In locoregional therapy, because the drug has to pass from the periphery to reach the tumor center, a major influential factor is the interstitium and interstitial fluid pressure. Interstitial pressure in tumors is usually increased. Because lymph flows from the tumor center toward the periphery, it flows in the opposite direction to peritoneally administered drugs [29]. Interstitium, or the so-called microenvironment, consists of collagen fibers linked through adhesion molecules, such as beta-1 integrins to fibroblast, parenchymal cells, and other interstitial cells. Emerging evidence suggests that giving drugs such as bortezomib, a proteasome inhibitor, can disrupt cell-cell adhesion, lower interstitial fluid pressure, and thus enhance drug diffusion into tumor tissue [30]. Some researchers tried to reduce the k constant with vasoactive agents able to regulate peritoneal-tissue blood flow, thus delaying drug clearance from the peritoneal cavity. Because these studies using vasoactive agents yielded conflicting data, mainly owing to the various experimental systems used, further studies are needed before vasoactive agents can be recommended [31].
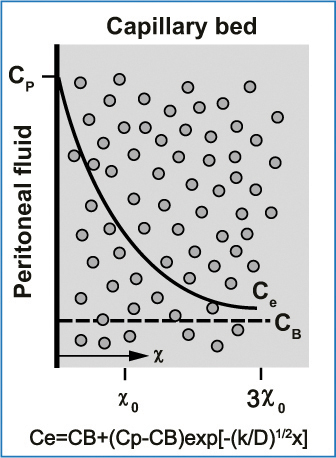

Fig. 8.7
Exponential decrease (solid line) in the free tissue interstitial concentration, Ce, as the drug diffuses down the concentration gradient and is removed by loss to blood perfusing the tissue. Also shown are the characteristics diffusion length, x0, at which the concentration difference between tissue and blood has decreased to 37 % of its maximum value, and 3×0, at which the difference has decreased to 5 % of its maximum value. Cp, free drug concentration in peritoneal fluid; CB, free drug concentration in blood (or plasma). (Reproduced from [28], with permission)
Another pharmacodynamic variable is hyperthermia. Hyperthermia unites the pharmacokinetic advantages inherent to intraperitoneal perfusion of cytotoxic drugs (regional dose intensification) with the direct cytotoxic effect induced by heat. Combining hyperthermia with intraperitoneally perfused chemotherapy agents increases tumor response through numerous mechanisms. Hyperthermia > 41°C inhibits DNA repair mechanisms in neoplastic cells, denatures proteins, induces lysosomal activation, and increases cell death [32, 33]. Hyperthermia also potentiates chemotherapy drug activity inhibiting intracellular drug-detoxification pathways and drug-induced DNA adduct repair mechanisms [34]. Last, hyperthermia helps drugs penetrate more deeply into tumor tissue. In a study investigating this hypothesis, Leunig et al. reported that heat induced a dosedependent reduction in interstitial pressure, thereby increasing drug penetration into tissues [35]. Pichè et al., using a murine model, showed that increasing the temperature for oxaliplatin HIPEC delivery increases tissue penetration without changing the pharmacokinetic advantages of the administration route and even reduces systemic toxicity [36]. Hyperthermia both induces and reverses certain forms of drug resistance, although the clinical importance of these interactions is still poorly understood. From a clinical point of view, the chance of reversing drug resistance using hyperthermia outweighs the dangers of inducing thermotolerance [37].
8.4 Bidirectional (Intraperitoneal plus Intravenous) Intraoperative Chemotherapy
Some have proposed bidirectional chemotherapy (intravenous plus endoperitoneal) drug infusion to obtain a bidirectional diffusion gradient in peritoneal neoplastic tissues. The first to propose this strategy was Elias et al., who suggested perioperative intravenous 5-fluorouracil (5-FU) and leucovorin in conjunction with oxaliplatin-based HIPEC [38], thus extending information from earlier studies suggesting that the two drugs induced a synergic effect [39, 40]. 5-FU is a thymidylate synthase inhibitor, enters the cell directly, and is then intracellularly metabolized to its active metabolite. Mild hyperthermia only slightly increases the 5-FU-induced effect, and the drug is not chemically compatible with other drugs in mixed solution. A study investigating the pharmacological rationale for perioperative 5-FU intravenous infusion during HIPEC in patients with PC from appendiceal cancer showed a definite pharmacologic locoregional advantage [41] (Fig. 8.8). The 5-FU concentration in peritoneal fluid is high 15 min after infusion, and this high drug level persists over 90 min. Single heated tumor nodules harvested at 15-min intervals showed 5-FU penetration. The study shows that perioperative bidirectional chemotherapy is pharmacokinetically, useful given that intravenous 5-FU application reaches a high concentration in the peritoneal fluid. By acting synergistically, bidirectional chemotherapy provides a high drug concentration in the tumor nodule. The bidirectional therapeutic approach is widely used by French and German centers for treating colorectal carcinomatosis [42, 43]. Similar results have been obtained after intravenous administration of ifosfamide (1,300 mg/m2 per liter continuous saline solution for 90 min) during HIPEC containing cisplatin and doxorubicin in a series of patients who underwent peritonectomy procedures for PC or mesothelioma [44]. This therapeutic strategy has enjoyed widespread use for various cancers at different time points (neoadjuvant or adjuvant) before or after surgical resection. Japanese investigators have obtained promising results by associating systemic and normothermic IP-CHT in a neoadjuvant setting in patients with advanced gastric cancers (GC) and neoadjuvant intraperitoneal and systemic (NIPS) therapy [45, 46]. Sugarbaker and Bijelic proposed using bidirectional (systemic and normothermic intraperitoneal) chemotherapy in an adjuvant setting to reduce locoregional recurrence after peritonectomy procedures plus HIPEC [47].
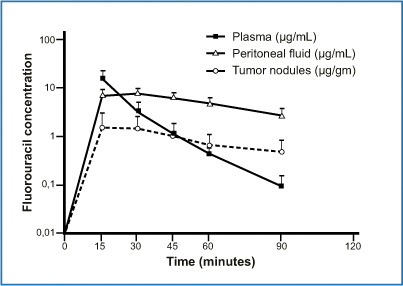

Fig. 8.8
Concentrations of 5-fluorouracil in plasma, peritoneal fluid, and tumor nodules after intravenous administration during hyperthermic intraperitoneal chemotherapy (HIPEC) procedure. (Reproduced from [41], with permission)
8.5 Overview of Drugs Commonly Used in Protocols for Perioperative Intraperitoneal Cancer Chemotherapy
8.5.1 Mitomycin C
Mitomycin C is an alkylating antibiotic (extracted from Streptomyces species) that acts mainly through DNA cross-linking. Mitomycin C has been extensively used in IP-CHT treatment protocols in appendiceal and colorectal carcinomatosis [48–50]. Although mitomycin is not regarded as a prodrug, it is not active against cancerous tissue as is the unchanged molecule. The drug changes into its active state as it enters the cell [51], and in vitro data suggest heat enhances mitomycin’s antitumoral activity [52]. Extending these findings, Jacquet et al. reported that intraperitoneally infused mitomycin C had a clear pharmacokinetic advantage over intravenous infusion, with an AUC IP/IV ratio of 23.5, findings confirmed by Van der Speeten [53, 54]. Drug dosimetry differs notably between the various research groups. Some institutions use mitomycin in a single dose, others a double dose, and still others a triple dose infused over 90 mins [55, 56]. A study from the Dutch Cancer Institute suggests that mitomycin at a dose of 35 mg/m2 yields the highest AUC IP/IV ratio with acceptable toxicity [55]. To maintain the concentration throughout the 90-min perfusion time, the dose was divided into three fractions: 50 % at the start, 25 % after 30 mins, and 25 % at 60 mins. Ample evidence describes the toxicity profile for mitomycin C, including anastomotic dehiscence and impaired wound healing [57, 58].
8.5.2 Cisplatin
Cisplatin is a well-known chemotherapy drug. It was the first member in the platinum family drug class, which now includes oxaliplatin and carboplatin. These platinum complexes react in vivo, binding to DNA and causing DNA crosslinking. Cisplatin induces cell death by causing DNA adducts to form [59]. The drug has been well studied for adjuvant IP-CHT in residual small-volume ovarian cancers (OC) after CRS. Three randomized trials showed a significant survival benefit [60–62]. For CRS plus HIPEC, cisplatin has been used for intracavitary therapy in patients with OC, GC, and malignant peritoneal mesothelioma (MPM). In their study, Urano and coworkers showed that cisplatin provided excellent in vitro and in vivo thermal augmentation [63]. Several groups have studied cisplatin penetration into tumor nodules. For example, Los et al. described intratumoral cisplatin distribution after intraperitoneal infusion and suggested that intraperitoneal cisplatin distribution reached its maximal advantage versus the intravenous route in the first 1.5 mm [64]. In a similar study, van der Vaart et al. investigated cisplatin-induced DNA-adduct formation and measured this drug-induced change 3–5 mm into the tumor tissue [65]. In an experimental model, Esquis et al. reported enhanced cisplatin penetration when they infused cisplatin at increased pressure [20].
8.5.3 Oxaliplatin
Oxaliplatin is a third-generation platinum compound that possesses a wide antitumor effect in vitro and in vivo, a better safety profile than cisplatin, and no cross-resistance with cisplatin or carboplatin. Oxaliplatin has a nonhydrolysable diaminocyclohexane (DACH) carrier ligand, which the final cytotoxic drug metabolites maintain. The bulky DACH ring retained by activated oxaliplatin is thought to cause formation of platinum-DNA adducts. These platinum-DNA adducts seem more effective than cisplatin adducts at blocking DNA replication and are more cytotoxic [66, 67]. The clinical use of oxaliplatin during bidirectional intraoperative chemotherapy in patients with PC was pioneered by Elias et al. [38, 68]. In a dose-escalation and pharmacokinetic study, they showed that 460 mg/m2 in 2 L/m2 of chemotherapy solution infused over 30 mins is well tolerated. The low AUC ratio is compensated by the rapid drug absorption into the tissue. In contrast to cisplatin and mitomycin, oxaliplatin is unstable in chloridecontaining solutions and can only be given in 5 % dextrose, a solution that may result in severe electrolyte disturbances and hyperglycemia during intracavitary therapy [69, 70]. A recent study using oxaliplatin in a murine model confirmed that heat increased its antitumoral activity [36]. In clinical practice, oxaliplatin given during HIPEC has proven activity in colorectal and appendiceal malignancies and has been used in patients with recurrent OC [71, 72].
8.5.4 Carboplatin
Carboplatin was introduced in the late 1980s and has since gained popularity in clinical treatment, inducing far fewer adverse effects than its parent compound, cisplatin. Carboplatin is a platinum compound with a higher molecular weight than cisplatin. Carboplatin is mostly used in intraperitoneal normothermic and, rarely, in hyperthermic chemotherapy protocols in patients with advanced OC [73, 74]. In a clinical study, investigators reported that normothermic carboplatin achieves acceptable bioavailability (calculated as AUC values), remaining at least six times higher in the intraperitoneal fluid than in the serum for 48 h [75]. Continuing research into bioavailability, Los et al compared the ability of carboplatin and cisplatin to penetrate into peritoneal cancer nodules in a rat model, and even though intraperitoneally administered carboplatin had a clear pharmacokinetic advantage over cisplatin, it proved far less able than cisplatin to penetrate into tumor cells [27]. A report showing opposite results has now revived clinical interest in intraperitoneally administered carboplatin [76].
8.5.5 Doxorubicin
Doxorubicin, or hydroxyl daunorubicin, is an antibiotic belonging to the anthracycline family. Although categorized as a DNA-intercalating drug, its true mechanism of action involves its critical interaction with the cell-surface membrane [77], and this interaction is influenced by temperature [78]. Given its wide in vitro and in vivo activity against a broad range of malignancies, its slow clearance from the peritoneal compartment due to the high molecular weight of the hydrochloride salt (579, 99 Dalton), its favorable AUC ratio of intraperitoneal to intravenous concentration times of 230, and the absence of risk for dose-limiting cardiotoxicity in intraperitoneal infusion, made doxorubicin a potential beneficial agent for perioperative intraperitoneal delivery. This advantage received support from experimental and clinical pharmacokinetic data [12, 13, 79]. Based on ex vivo studies, Pilati et al. suggested that during hyperthermia, doxorubicin uptake increases, and tumor cells become more sensitive to the drug [80]. Pegylated liposomal doxorubicin has generated interest for HIPEC because of its favorable pharmacokinetics [81]. Doxorubicin-based HIPEC has been used in peritoneal surface malignancy from appendiceal, GC, OC, and colon cancer, and MPM.
8.5.6 Gemcitabine
Gemcitabine is a nucleoside analog in which the hydrogen atoms on the 2’ deoxycytidine carbon are replaced by fluorine atoms. As with pyrimidines, the triphosphate gemcitabine analog replaces one of the nucleic acid building blocks, in this case cytidine, during DNA replication. This process arrests tumor growth, because only one additional nucleoside can be attached to the “faulty” nucleoside, thus resulting in cell death. Another gemcitabine target is the enzyme ribonucleotide reductase. Gemcitabine exerts widely ranging in vitro and in vivo cytotoxic activity, particularly against pancreatic and lung cancer. To extend knowledge on its actions, Pestieau et al. investigated intraperitoneally delivered gemcitabine pharmacokinetics and tissue distribution in a rat model [82]. The AUC ratio (IP/IV) after intraperitoneal infusion was 26.8 ± 5.8 favoring intraperitoneal use. Several investigators explored the use of normothermic intraperitoneally delivered gemcitabine in advanced cancer outside the setting of CRS [83, 84]. Resected advanced pancreatic cancer at high risk of locoregional recurrence is a potential indication for intraoperative intraperitoneally delivered heated gemcitabine in an adjuvant setting [85–87].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree