Study [Reference]
Year
Patients
Drug
Temperature (°C)
Duration
Mortality
Morbility
Survival
Yan et al. [22]
2007
34
CDDP 120 mg + MMC 30 mg
42.5–43.5
60–90
–
14.7%
3 yrs 5.9% (CC, 0–1, 12 mo)
Glehen et al. [23]
2010
159
MMC 30–50 mg/m2 + CDDP or LOHP 340–460 mg/m2+ Irinotecan 100–200 mg/m2
41–43
30–120
6.5%
27.8%
5 yrs 5.9% (CC, 23%)
Yang et al [24]
2010
28
CDDP 120 mg or HCPT 20 mg + MMC 30 mg
42.5–43.5
90–120
0%
14.3%
2 yrs 42.8% (CC0, 43.4 mo)
Yonemura et al. [25]
2005
107
CDDP 300 mg Etoposide 150 mg MMC 30 mg
42–43
60
2.8%
21.5%
5 yrs 6.7% (CC0, 13% CC > 0,2%)
Glehen et al [26]
2004
49
MMC 10 mg/L
45–48
90
4%
27%
5 yrs 16% (CC0–1, 29.4%)
In the world literature, nine studies reported a total of 24 treatment-related deaths from a total of 467 patients, an overall mortality rate of 5.1% and morbidity of 21.5% (Table 18.2) [23, 27–34]. A recent review of CRS plus HIPEC for treating PC of any origin reported a mean mortality rate of 2.9%, with tertiary centers reporting a mortality rate ranging from 0.9% to 5.8% [35]. Combined morbidity was reported to be 28.8 %; the most common complications following CRS plus HIPEC were abscesses, fistulas, and anastomotic leaks [36]. CRS plus HIPEC for PC from GC has comparatively similar mortality and morbidity rates as for PC of other organ origins.
Table 18.2
Mortality rates of CRS plus HIPEC
Study [Reference] | Treatment-related deaths | Mortality (%) | Cause of death |
|---|---|---|---|
Glehen et al. [23] | 11 | 6.5 2 | MOFs , 2 septic shocks, 1 fistula, 1 PE, 1 CVA, 1 toxicity |
Yang et al. [27] | 3 | 10.7 | 2 ileus, 1 ARDS, 1 pneumonia |
Scaringi et al. [28] | 1 | 11 | Septic shock |
Roviello et al. [29] | 1 | 1.6 | MOF |
Farma et al. [30] | 1 | 5.6 | CVA |
Yonemura et al. [31] | 3 | 7 | 1 ARF, 1 A-leak, 1 bleeding |
Mussa et al. [32] | 1 | 14.3 | |
Fujimura et al. [33] | 0 | 0 | |
Beaujard et al. [34] | 3 | 3.6 | 1 PE, 1 MOF, 1 septic shock |
Total; median | 24 | 5.1 |
Since PC from GC is essentially a fatal disease with conventional treatment options, these mortality and morbidity rates may be acceptable to patients. The importance of achieving CC is emphasized in the literature, with a twofold increase in MS with a CC score of 0 or 1. This suggests that CRS plus HIPEC for PC from GC should only be considered in select patients if the surgeon is very confident that a CC-0 is possible. The overall morbidity rate was reported by eight authors to be 21.5% (Table 18.3) [27–31, 33, 34]. CRS plus HIPEC may improve survival in select patients with with PC from GC: MS is increased to 15 months in patients with CC-0 compared with 3 months with only basic supportive therapy.
Table 18.3
Morbidity and complications of CRS plus HIPEC for PC from GC
Study [Reference] | Overall morbidity (%) | Reoperation (%) | Sepsis | Fistula | Abscess | Hematologic toxicity | Ileus | Anastomotic leak |
|---|---|---|---|---|---|---|---|---|
Yang et al. [27] | 14.3 | 1 | 1 | 2 | 1 | |||
Scaringi et al. [28] | 27 | 9 | 5 | 2 | ||||
Roviello et al. [29] | 27.9 | 8.2 | 5 | 2 | 5 | 1 | ||
Farma et al. [30] | 55.6 | 1 | 3 | |||||
Yonemura et al. [31] | 21.5 | 1 | 6 | 6 | ||||
Fujimura et al. [33] | 50 | 33.3 | 2 | |||||
Beaujard et al. [34] | 9.6 | 4.8 | 3 | 2 | 1 | |||
Median | 21.5 |
18.2 Role of Systemic Chemotherapy in Peritoneal Carcinomatosis from Gastric Cancer
PC from GC is typically treated with systemic chemotherapy; however, its efficacy is difficult to determine based on the literature. Three clinical trials found that systemic chemotherapy improved MS in metastatic GC to 7–10 months; however, patient populations were heterogeneous and inconsistently randomized, with the majority having no PC [5–7]. Similarly, Preusser et al. [37] reported decreased response rates to systemic chemotherapy in patients with PC. No clinical trials have directly compared CRS plus HIPEC vs. systemic chemotherapy in patients with PC from GC.
18.3 Role of HIPEC in Peritoneal Carcinomatosis from Gastric Cancer
Tumor extent in the gastric serosa and lymphatic spread are the two most important factors affecting prognosis in patients with GC [38–40]: when the gastric serosa is infiltrated, PC occurs frequently [41] (Fig. 18.1). In this condition, ∼ 50% of patients with advanced GC (AGC) will develop PC despite undergoing radical surgery. PC is also common in GC, being already present in 5–20% of patients explored for potentially curative surgery.

Fig. 18.1
Gastric cancer with serosal invasion
The presence of free peritoneal tumor cells (FPTC) in washing specimens were identified in up to 24 % of patients with GC stage Ib and up to 40 % in stages II and III [41]; in 10–38% of cases, the peritoneum was the only site of recurrence [42–46] (Fig. 18.2).
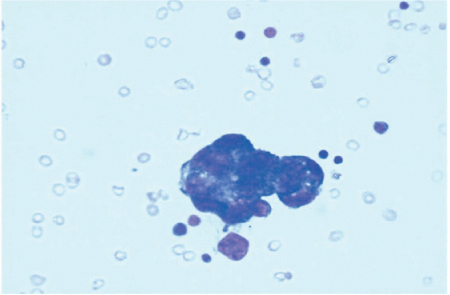

Fig. 18.2
Free peritoneal tumor cells (FPTC) in washing specimen
The 5-year survival rate in patients with PC from GC is < 3% [47], with an overall mean and MS of 6.5 and 3.1 months, respectively [34]. Saito et al. [48] reported a 5-year survival rate of patients with advanced GC with FPTC of 15.3 %, similar to that of patients having macroscopical peritoneal metastasis (14.8 %).
Systemic chemotherapy may improve MS up to 12 months in advanced/metastatic GC, but a similar survival benefit has not been reported in macroscopic PC. Yonemura et al. [49] affirmed that the use of adjuvant systemic chemotherapy after radical resection in patients with FPTC showed a survival benefit. Patients treated with adjuvant chemotherapy survived significantly longer than patients in the control group: the 1- and 2-year respective survival rates were 88 % and 44 % in the adjuvant group and 53 % and 9 % in the adjuvant group and the control group. Neoadjuvant chemotherapy (NACT) has been described to decrease the load of macroscopic PC from GC [50]. Yano et al. [51] reported a small series of four of 26 patients (15.4 %) affected by PC from GC who attained complete remission of peritoneal metastasis after NACT. All these patients subsequently underwent curative resection. Inokuchi et al. [52] reported a partial response in nine of 13 patients (69 %). However, a further study suggested that after NACT, detecting FPTC can change from positive to negative and vice versa: ten of 42 patients (24 %) with negative peritoneal cytology shifted to positive for FPTC during NACT, whereas seven of 19 (37 %) with FPTCpositive cytology at staging laparoscopy turned negative.
GC peritoneal spread remains a major problem, and some authors finally suggest that there is no role for surgery in these instances.
As with other types of PC, in PC from GC, HIPEC after CRS is performed to eliminate FPTC and prevent or delay PC [53]. A number of studies have been conducted with the aim of demonstrating a significant reduction in the rate of subsequent PC and an increase in survival of patients with AGC when radical surgery was combined with HIPEC [22, 54–57]. Yonemura et al. [58] demonstrated that HIPEC could significantly improve the MS from 15 to 48 months and the 5-year survival rate from 12 % to 42 % in patients with FPTC. However results of CRS plus HIPEC treatment of PC from GC seem to be less encouraging in terms of survival, morbidity, and mortality when compared with PC from other tumor types [59–61]. In a French retrospective multicenter study, the PC from GC group showed the worst outcome, with 3- and 5-year survival rates of 18 % and 13 %, respectively. In 2010, Li et al. [62] reported a series of 128 patients with PC from GC: the MS in the unresected group was 6 months, compared with 11.8 months for resected patients. Moreover, the authors observed a significantly improved survival in patients treated with surgery plus HIPEC compared with those treated with surgery alone, but postoperative complications were more frequent in HIPEC cases than in patients with resection alone (20.0 % vs. 13.2 %, P = 0.34). Yang et al. [27] published the final results of a phase III randomized trial to evaluate efficacy and safety of CRS plus HIPEC for treating PC from GC. Median overall survival (OS) was 6.5 months in the CRS-alone group and 11 months in the CRS plus HIPEC group (P = 0.046). This outcome was even more significant in patients with synchronous PC from GC (n = 51): median OS was 12 months in the CRS plus HIPEC group (n = 24) and 6.5 months in the CRS group (n = 27, P = 0.029). The 1-, 2-, and 3-year survival rates were, respectively, 29.4 %, 5.9 %, and 0 % for CRS group and 41.2 %, 14.7 %, and 5.9 % for the CRS plus HIPEC group.
CC score has been demonstrated to influence survival, but HIPEC obtained a significant advantage in patients with CC 0-1 compared with those with CC 2–3. Multivariate analysis recognized CRS plus HIPEC, synchronous PC, CC 0-1, systemic chemotherapy, and no serious adverse events as major independent predictors for better survival. HIPEC was ∼ 2.6 times more likely to increase survival. In a systematic review, Gill et al. [36] analyzed survival, mortality, and morbidity in the treatment of PC from GC with CRS plus HIPEC: overall MS was 7.9 months. In the subgroup of patients with residual nodules after CRS < 0.25 cm in diameter, MS rose to 15 months. The 1- and 5-year survival rates were 43 % and 13 %, respectively. The treatment-related mortality rate was 4.8 % and morbidity 21.5 %.
In conclusion, in PC from GC, CRS plus HIPEC proved, with good evidence, to improve survival, with acceptable morbidity and mortality rates. It is extremely important to obtain the diagnosis and the diffusion grade of PC from GC before CRS plus HIPEC with the use of staging laparoscopy. The role of surgery is fundamental; CC was strictly related to improved survival. In patients with PC from GC, multimodal treatment is mandatory, with the pivotal role being HIPEC after CRS.
18.4 Surgical Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy
Two recent studies reported MS times > 15 months in patients with PC from GC treated with CRS plus HIPEC [24]. Importantly, both studies also reported CC score as an independent prognostic factor for survival. The experience of institutions and surgeons performing CRS plus HIPEC shows that CC seems to be associated not only with survival but with both mortality and morbidity. The multi-institutional study by Glehen et al. [23], which comprised 159 patients from 15 institutions, reported that the institution at which CRS plus HIPEC was performed was an independent prognostic factor of postoperative complications. CRS plus HIPEC are considered technically challenging procedures with steep learning curves. Smeenk et al. [63] performed CRS plus HIPEC for PC over a 10-year period and analyzed the rate of CC and postoperative morbidity over three consecutive treatment periods. They reported a significantly increased CC rate from 35.6 % to 65.1 % and a subsequent decreased postoperative morbidity from 71.2 % to 34.1 %. Furthermore, they reported that the peak of the learning curve was reached after 130 procedures. Yan et al. [64] compared morbidity rates following CRS plus HIPEC for peritoneal surface malignancies (PSM) in 140 consecutive patients. They reported that severe morbidity rates decreased from 30 % to 10 % when comparing the last 70 cases with the first 70 cases. Controversy remains regarding the treatment of patients with PC from GC: systematic review demonstrates similar mortality and morbidity rates for CRS plus HIPEC for PC from GC compared with PC from other organs. Survival improved in these patients compared with basic supportive therapy; however, systemic chemotherapy data specifically for this population is scarce.
A recent trial comparing gastrectomy, metastasectomy, plus systemic therapy versus systemic therapy alone (GYMSSA trial) was recently conducted in patients with GC [65]. As a prospective phase III randomized trial, GYMSSA has the potential to clarify whether an aggressive surgical approach combined with HIPEC and systemic chemotherapy may benefit GC patients. However the trial included patients with limited metastatic disease, including lung and liver metastases, while HIPEC focused on PC from GC without evidence of distant metastases.
18.5 Adjuvant Role of Hyperthermic Intraperitoneal Chemotherapy
Peritoneal metastasis is the most common type of recurrence and cause of death after surgery in patients with GC [66], developing in ∼ 20–50 % of patients who undergo a curative gastrectomy and increasing to 80 % for those with positive peritoneal cytology [67–69]. Tumour-positive cytology has been clearly correlated with intraperitoneal (IP) recurrence and is significantly associated with decreased disease-free (DFS) and OS rates [54, 55, 70] Intravenously administered chemotherapy plus radiotherapy showed no significant survival advantage as adjuvant treatment for patients with a high risk of PC from GC.
Several studies [29, 71–73], including a number of randomized clinical trials (RCTs), have been performed to investigate the usefulness of HIPEC in a prophylactic setting or as adjuvant treatment after potentially curative GC resection. The RCTs conducted by Koga et al. [74] reported a considerably higher 3-year survival rate of patients in the HIPEC plus surgery group (83.0 %) compared with those in the control group (67.3 %), although this was not statistically significant. Similar results were obtained from RCTs reported by Hamazoe et al. [75], with a lower incidence of peritoneal recurrence and a higher 5-year survival rate of patients in the HIPEC group compared with the control group (64.2 % vs. 52.5 %), although the survival benefit did not achieve a statistically significant difference. The RCT conducted by Fujimoto et al. [76] was the first to demonstrate significantly improved peritoneal recurrence and long-term survival rates in the surgery plus HIPEC group compared with surgery alone after curative resection: in addition, peritoneal recurrence rate in the HIPEC group was significantly lower (p < 0.0001) compared with that of the control group. In their randomized trials, Fujimura et al. [33] and Yonemura et al. [77] showed a significantly higher rate of survival after adjuvant HIPEC combined with surgery compared with surgery alone: their results showed the independent and synergistic effect of hyperthermia, along with chemotherapeutic agents, against cancer cells, which seems to increase greatly at temperatures of 42.5°C–43°C. Yonemura et al. [31] more recently confirmed similar results in an RCT involving normothermic intraperitoneal chemotherapy (NIPEC) procedures: the 5-year survival rate of patients treated with the combination of HIPEC and surgery was significantly higher, at 61 %, than those of the other two groups (NIPEC plus surgery and surgery alone).
Overall, randomized clinical trials on adjuvant HIPEC procedures demonstrate that the peritoneal recurrence rate decreased and survival improved with this treatment modality in patients with advanced GC.
Xu et al. [78] reviewed all available randomized trials investigating the use of HIPEC after radical, potentially curative, resection for locally advanced GC: the authors found seven RCTs comparing surgery plus HIPEC with surgery alone. Based on their results, HIPEC benefitted the patient after curative resection versus resection alone, and in particular, the combination of HIPEC or activated carbon particles was superior to other forms of HIPEC. Similarly, Yan et al. [79] reviewed all clinical trials studying the adjuvant role of perioperative IPEC in resectable GC: based on the meta-analysis of the pooled data from 1,648 patients, a significant improvement in survival was noted with HIPEC alone or HIPEC plus EPIC. A trend toward survival improvement with normothermic IPEC (NIPEC) was also shown, whereas there was no significant trend with either EPIC alone or HIPEC.
18.6 Discussion
The combination of CRS plus HIPEC is to date the only therapeutic strategy with a hope of long-term survival at 5-years in patients with PC of GC. HIPEC could be performed with the aim of minimizing the rate of peritoneal recurrences (adjuvant intent), but several RCTs confirm the survival advantage and the potential adjuvant role of HIPEC in preventing PC in advanced GC.
Patients with serosa-invasive tumors or positive peritoneal cytology are the high-risk groups that show a significant risk of PC and very poor survival prospects: these groups can reasonably be considered for HIPEC and may particularly benefit from this treatment for preventing peritoneal recurrence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree