Fig. 21.1
Proposed models for the development of types 1 and 2 tumors of the ovary. SCOUT, secretory cell outgrowths; P53, tumor protein 53; STIC, serous tubal intraepithelial carcinoma; PPSPC, primary peritoneal serous papillary carcinoma; HGSC, high-grade serous cancer; LGSC, low-grade serous cancer. (Modified from [11])
Type I tumors comprise low-grade serous, low-grade endometrioid, mucinous, and clear-cell carcinomas. These tumors typically present as large cystic masses confined to one ovary, have a relatively indolent course, are genetically relatively stable, and typically display various specific mutations, including KRAS, BRAF, PTEN, PIK3CA, CTNNB1, ARID1A, and PPP2R1A genes. These molecular alterations result in morphologic changes that progress in a stepwise manner from benign through varying degrees of atypia (borderline tumor) to noninvasive and then invasive carcinoma. Traditional thinking maintains that these low-grade precursor lesions correlate with epithelial inclusion cysts and derive from ovarian surface epithelium invaginations that later, owing to estrogen hormone stimulation, progress to Müllerian type metaplasia [12]. Observations from a study conducted by Li et al. [13] show that most epithelium lining inclusion cysts are immunohistochemically and phenotypically PAX8(+), closely resembling tubal epithelium. Given the close anatomical relationship between the fimbriae and the ovarian surface, when ovulation disrupts the ovarian surface epithelium, tubal mucosa may implant on the ovary and become entrapped within the ovarian cortex [14]. Hence, according to a putative evolutionary model [10], papillary tubal hyperplasia (PTH) of the tubal mucosa through KRAS/BRAF mutations may be responsible for the development of atypical proliferative serous tumors (APST) and endosalpingiosis. As they progress further, APST subsequently develop into serous borderline tumor and invasive low-grade serous carcinoma.
Type II tumors comprise high-grade serous carcinoma (HGSC), high-grade endometrioid carcinoma, malignant mixed mesodermal tumors (carcinosarcomas), and undifferentiated carcinomas. These tumors are usually in an advanced stage at onset (> 75??%), grow rapidly, and are highly aggressive. Type II tumors are chromosomally highly unstable, in more than 95 % of cases harbor TP53 mutations, and rarely display mutations found in the type I tumor. BRCA is inactivated in up to 40–50% of HGSC [6]. During the past few years, abundant histological and molecular evidence has emerged that strongly suggests that most high-grade ovarian and peritoneal serous carcinoma originate in the fallopian tube, specifically from a serous tubal intraepithelial carcinoma (STIC), thus implying that ovarian and peritoneal spread is metastatic [15–17]. Despite possible exceptions, including a rare serous borderline tumor evolving into highgrade serous carcinoma [18], histological and molecular evidence shows that nearly all ovarian and peritoneal high-grade serous carcinomas derive from STIC and arise in the fallopian tube. Tubal carcinogenesis manifests with increased positive immunohistochemical staining for nuclear p53 in the tubal secretory epithelium (so-called p53 signature), which would evolve into STIC characterized by increased positive staining for Ki-67 up to overt HGSC with a TP53 gene mutation [5].
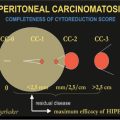
These histogenetic theories imply that the term pelvic serous carcinoma might be more appropriately applied to describe a single disease characterized by a high-grade serous carcinoma, an entity previously differentiated into PPSPC, tubal cancer, and OC. These findings notwithstanding, the literature on PPSPC in recent years contains numerous case reports, some referring to men [19, 20], and a few case series, most including no more than 10–20 patients [21, 23]. Apart from classification problems, the therapeutic principles overall resemble the treatment schemes applied for OC, the same staging procedures apply [International Federation of Gynecology and Obstetrics (FIGO)], and studies analyzing treatment results or data for follow-up procedures usually combine the two types [24–28]. Equally important, studies conducted in recent years and designed to compare PPSPC (identified according to the GOG criteria) and serous OC, have, as expected, failed to identify substantial differences [22, 29]. Outcome data for patients with PPSPC treated according to GOG criteria and provided by the major international studies [21–23, 29], including experience obtained in our own department (Table 21.1), dictate the following conclusions. First, the advanced intra-abdominal spread already present when PPSPC becomes clinically manifest makes published results extremely difficult to assess without quantifying the amount of disease present at the first therapeutic approach. As Table 21.1 shows, the only groups who assessed this variable with an adequate score [the Peritoneal Cancer Index (PCI)] are research centers used to treating peritoneal surface malignancies. Hence, only they can provide this descriptive variable, without which even the FIGO classification fails to provide all the necessary information. Another essential point to emphasize again, especially given the new histogenetic theories on OC, is that a disease resulting in severe peritoneal spread cannot be staged with the FIGO classification given that FIGO staging considers together in the subgroup IIIc patients with severe peritoneal spread along with those with minimal peritoneal spread and a single lymph-node metastasis [30, 31]. Any disease-staging system that omits the PCI risks losing much information. A second concern is that these patients often undergo preoperative neoadjuvant chemotherapy. Even though the precise role of neoadjuvant chemotherapy in these neoplasias and in the so-called OCs remains undefined [32], peritoneal spread can make complete cytoreduction hard to achieve, so that an optimal surgical outcome means recourse to downstaging chemotherapy. Earlier studies have already used this approach in patients with PPSPC [33]. A final possible concern regards the therapeutic procedures depending on the clinical characteristics of OC. Obviously in these cases, the definition used for surgical cytoreduction, albeit integrated with the adjective “optimal” (a term that apart from anything else foresees substantial residual disease), provides few elements for judgment. In a disease such as PPSPC, inherently involving extensive peritoneal involvement, the only approach that seems to answer the need for a rational therapeutic program is to use peritonectomy procedures, hence defining the extent of surgical cytoreduction obtained according to standardized criteria [Completeness of Cytoreduction (CC) score]. Among the studies we review in Table 21.1, the only series to report obtaining in most patients a percentage of complete cytoreduction without macroscopically evident residual disease, thus yielding an acceptable outcome, is the case series collected by Bakrin et al. [23] and data collected in our department. A final consideration merits integrating the peritonectomy procedure with hyperthermic intraperitoneal chemotherapy (HIPEC), as generally done when surgery ends in centers specialized in treating peritoneal surface malignancy [34]. Although this combined approach is now considered standard care for treating pseudomyxoma peritonei (PMP) [35], malignant peritoneal mesothelioma (MPM) [36], and selected patients with colorectal carcinomatosis [37], HIPEC combined with cytoreduction surgery (CRS) remains highly controversial in patients with peritoneal spread from gynecological malignancies, and the controversy concerns HIPEC itself [38]. The chief objection regards a possible increase in morbidity and the lack of randomized controlled studies to assess the eventual benefits accruing from HIPEC plus CRS. While awaiting the results of numerous ongoing trials, to which this book devotes a specific chapter, the increasingly encouraging results obtained with normothermic adjuvant intraperitoneally applied chemotherapy (IP-CHT) seem to envisage an ever growing space for using HIPEC when surgical cytoreduction ends, especially insofar as the two techniques, rather than being alternatives, could be complementary [39, 40].
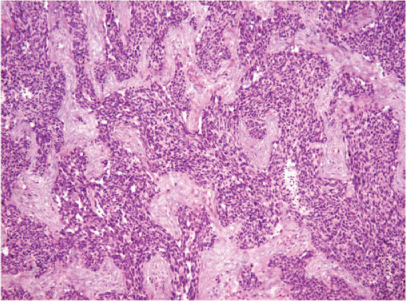
Table 21.1
Literature review
Study [Reference] | No. cases | Age (years) | Stage of disease | Surgical procedures | Cytoreduction obtained | Pre-operative chemotherapy | Survival |
|---|---|---|---|---|---|---|---|
Liu et al. 2011 [21] | 22 | 56.2 | FIGO IIIC (54.5%) | Pelvic peritone- ctomy + lymphadenectomy | – | 22.7% | 21 months |
Kawaguchi et al. 2012 [29] | 22 | 67 | – | Pelvic peritonectomy + lymphadenectomy | Optimal cytoreduction < 1 cm, 54.5% | 90.9% | 26.5 months |
Chao et al. 2013 [22] | 38 | 63 | FIGO IIIC (81.6%) | Cytoreduction NOS | Optimal cytoreduction < 1 cm, 71.9% | – | 62 months |
Bakrin et al. 2013 [23] | 36 | 60.5 | Median PCI 10 | PRT + HIPEC | Complete cytoreduction 75% | 97.2% | 5- year OS 57.4% |
Di Giorgia (unpublished data) | 12 | 59.7 | Median PCI 14.8 | PRT + HIPEC | Complete cytoreduction 75% | 50% | 61 months |

Fig. 21.2
Desmoplastic small round cell tumor (DSRCT)
21.3 Desmoplastic Small Round Cell Tumor
Desmoplastic small round cell tumor (DSRCT) is a rare and highly aggressive neoplasm involving children, adolescents, and young adults that begins and spreads on the peritoneal surface. Approximately 300 DSRCT cases have been reported in the literature since the tumor was initially described by Gerald and Rosai in 1989 [41]. Histologically, DSRCT consists of desmoplastic stroma containing small round blue cells in nests (Fig. 21.2) and is associated with a characteristic chromosomal translocation, t(11;22)(p13;q12), which fuses the N-terminus of the Ewing sarcoma (EWS) gene to the C-terminus on the Wilms tumor (WT-1) gene [42, 43]. The reference standards for diagnosis include cytogenetic evaluation to confirm the characteristic translocation and EWS-WT-1 fusion. DSRCT usually arises from abdominal or pelvic peritoneum as a diffuse multifocal disease similar to carcinomatosis, even though it sometimes arises also in solid organs, such as ovaries, liver, kidneys, pancreas, and brain [44]. Patients typically present in an advanced stage with multiple peritoneal-based lesions and, despite multiple strategies that include several chemotherapy regimens, aggressive debulking surgery, and whole abdominal radiation, durable remission remains rare, with a 15 % 5-year overall survival [45]. Current guidelines include neoadjuvant chemotherapy, debulking surgery, adjuvant chemotherapy, and radiation therapy. The MD Anderson Cancer Center group was the first to apply the HIPEC procedure when satisfactory (CC-0/1) cytoreduction ended [46]. The standard regimen is dose-intense neoadjuvant chemotherapy (recently combined with irinotecan and bevacizumab) with cyclophosphamide, doxorubicin, vincristine alternating with ifosfamide, and etoposide [47, 48]. According to the MD Anderson Cancer Center, case series patients were evaluated after receiving chemotherapy for 4–6 months; those having a partial response and found to be candidates for complete cytoreduction underwent surgery including HIPEC using cisplatin at 100 mg/m2 with a maximum dose of 130 mg for 90 min [45]. One of the main prognostic factors is the completeness of surgical resection. In their Cox regression analysis, Zhang et al. identified this variable as the only independent outcome indicator (Table 21.2) [49]. After chemotherapy and maximal surgical debulking, multimodal protocols included whole-abdominopelvic radiation therapy (WAP-RT) (30 Gy) with or without focal boost according to the minimal residual disease eventually present [46]. A recent report from the Memorial Sloan-Kettering Cancer Center shows that intensity-modulated radiation therapy (IMRT) for consolidative WAP-RT after surgical debulking reduces toxicity and improves the therapeutic index [50].
Table 21.2
Cox regression analysis for overall survival in desmoplastic small round cell tumor (DSRCT) patients. (Reproduced from [49], with permission)
Parameter | SE | OR | 95 % CI | P value |
|---|---|---|---|---|
Age | .442 | .587 | 0.247–1.397 | .228 |
Gender | .496 | .986
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|