Summary of Key Points
- •
Concerning radiotherapy, higher physical or biologic dose (altered fractionation) is associated with better local control and, in some trials, with better survival. Current evidence favors a schedule of 60 Gy to 66 Gy in 6 weeks to 7 weeks, with no benefit for doses beyond that.
- •
Concurrent chemoradiation therapy is the optimal treatment strategy with curative intent for fit patients not candidates for surgery.
- •
Currently, there is no place for adding a molecularly targeted agent to the combined-modality regimens outside a clinical trial, which should select patients based on the relevant biomarker.
- •
The choice between surgery and chemoradiotherapy should be discussed within a multidisciplinary tumor board based on patient comorbidity and preferences, and prognostic factors.
- •
Prophylactic cranial radiation is not recommended as a standard therapy.
Stage III disease accounts for one-third of all lung cancers and comprises the most heterogeneous group of tumors in terms of clinical presentation and treatment options. Two articles published in 2012 highlight the debate and controversy about managing stage IIIA nonsmall cell lung cancer (NSCLC). Among thoracic surgeons surveyed, 84% of thoracic surgeons favored neoadjuvant therapy followed by surgery for microscopic N2 disease. For grossly involved N2, 62% of these surgeons favored neoadjuvant therapy followed by surgery in the context of mediastinal downstaging but only 32% choose this approach for bulky disease. In a survey of oncologists, 92% favored a neoadjuvant approach followed by surgery for minimal N2 and 52% chose chemoradiation therapy for bulky disease. Treatment options for NSCLC range from aggressive use of a single modality through to trimodality treatment that includes surgery, chemotherapy, and radiotherapy.
Significant advances in treatment have improved outcomes for patients with locally advanced NSCLC since the 1980s. Active areas of research include determining the appropriate sequence of systemic treatments, discovering novel agents, and improving delivery of radiotherapy through technologic advances. Current treatment paradigms extend beyond age, performance status, and nonsmall cell histology and incorporate an expanding list of factors in the decision-making process. In the near future, therapeutic strategies will be individualized based on the identifiable molecular characteristics of a tumor, leading to better patient outcomes and more effective clinical trial design. Technologic improvements in radiotherapy enable oncologists to target tumors with more precision and effectiveness, thus making it an option for patients who previously might have not been candidates for this treatment modality.
Definitive radiotherapy had been the standard of care for patients with locally advanced NSCLC until results from clinical trials showed that chemoradiation therapy improved survival. (When considering the trials reviewed in this chapter, it is important to remember that the old tumor, node, metastasis [TNM] classification system—the sixth edition—was usually used.)
Radiation alone is the definitive treatment for patients with locally advanced NSCLC who are not candidates for chemoradiation therapy. Radiotherapy also has a role in the treatment of select patients with isolated thoracic recurrence. Benefits of radiotherapy include palliation of tumor-related symptoms, local control of tumor growth, and a potential survival advantage.
Radiotherapy Dose and Fractionation
Dose
When radiotherapy alone is used to treat locally advanced NSCLC, the median survival is approximately 10 months and the 5-year survival rate is 5%. In the 1970s, the Radiation Therapy Oncology Group (RTOG) conducted a phase III trial (RTOG 73-01) to evaluate the effect of radiotherapy dosage on local control rates and overall survival. Patients were randomly assigned to treatment with 40 Gy, 50 Gy, or 60 Gy in 2-Gy daily fractions or to a split-course schedule. Local control rates were significantly better with the highest dose (52% vs. 62% vs. 73%, respectively; p = 0.02), although median survival rates were similar (10.6 months vs. 9.5 months vs. 10.8 months, respectively). The split-course schedule was associated with inferior local control and survival. This trial established 60 Gy in 30 fractions as the standard radiotherapy dose-fractionation scheme for decades.
Early radiotherapy portals were designed to cover the primary tumor, ipsilateral hilum, ipsilateral and contralateral mediastinum, and ipsilateral supraclavicular nodes, leading to a large irradiated volume. This approach was called elective nodal irradiation. As the toxicity of this approach and the relation between local failure occurring mainly at the level of the gross tumor volume and poor patient outcomes became more apparent, treatment planning shifted toward involved field radiation. Concern about the potential for nodal recurrence has slowed the adoption of involved field radiation; however, a prospective randomized trial from China showed promising results. Patients with locally advanced NSCLC were treated with 68 Gy to 74 Gy involved field radiation or 60 Gy to 64 Gy elective nodal irradiation. At 5 years, patients who received involved field radiation had significantly better overall response rates (90% vs. 79%, p = 0.032), local control (51% vs. 36%, p = 0.032), and fewer cases of pneumonitis (17% vs. 29%, p = 0.044).Treatment with involved field radiation significantly improved overall survival at 2 years (39.4% vs. 25.6%, p = 0.048). Despite several limitations of this study, the results are intriguing and suggest that involved field radiation is unlikely to compromise clinical outcomes. Furthermore, several studies have clearly demonstrated that the number of isolated nodal failures outside the involved field radiation remains very low.
Technologic advances have enabled researchers to determine the optimal volume and explore the role of dose escalation in improving local control rates. The introduction of positron emission tomography (PET)–computed tomography (CT) imaging has enhanced treatment planning. The addition of cone-beam CT on linear accelerators has led to new radiotherapy such as intensity-modulated radiotherapy (IMRT)—either static or rotational—and image-guided radiotherapy, which improves the accuracy of daily radiotherapy delivery. Because of these improvements, the classical safety margins can be decreased, allowing researchers to increase the total dose either physically or biologically.
In early phase I/II trials, increasing the radiotherapy dose to 74 Gy or more improved the median survival times to 24 months. Given the promising results of these trials and a pooled analysis of Cooperative Group studies, a phase III randomized trial (RTOG 06-17 trial) was designed to compare concurrent chemoradiotherapy and dose-escalated radiotherapy with standard radiotherapy dosage. There was a second randomization to evaluate the role of cetuximab. Patients with locally advanced NSCLC were randomized to a standard-dose radiotherapy (60 Gy in 30 daily fractions) or a high-dose radiotherapy (74 Gy in 37 fractions) concurrently with weekly paclitaxel and carboplatin followed by two cycles of consolidation and to cetuximab or not. The 2-year survival rates were 58% for the standard dose and 45% for the high radiation dose. The local failure rate was also higher in the experimental arm: 38.6% versus 30.7%, respectively, at 2 years. Planning target volumes were very similar between the two arms as well as the use of IMRT. However, although 10 patients died in the 74-Gy arms compared with two in the 60-Gy arms, the toxicity rates were not different between the two groups. Several explanations have been put forward to explain these worse outcomes in the higher dose arm, including heart toxicity and the loss of efficacy through longer overall treatment time and accelerated repopulation. It is important to note that the outcomes in the low-dose arm are among the best ever observed in a population with stage III NSCLC. A subsequent analysis examined the role of IMRT as patients were stratified according to the radiation technique: the planning target volume was larger for patients treated by IMRT compared with three-dimensional conformal radiation therapy (486 mL vs. 427 mL), but the outcomes were similar for the two techniques. Less grade 3 pneumonitis, lower heart dose, and less dose reduction for chemotherapy were observed for patients treated by IMRT. There was a concern that IMRT could result in very low doses of radiation to large volumes of normal lung with increased pneumonitis risk, but an increased incidence of radiation pneumonitis was not observed.
Altered Fractionation Schedules
Multiple trials have tested the use of altered dose-fractionation schedules to improve the therapeutic index of radiotherapy. These approaches have included hyperfractionation (two or three fractions per day with a lower dose per fraction over the standard treatment duration), accelerated fractionation (use of a standard fraction size and total radiation dose, given over a shorter overall time), or a combination of these approaches. Compared with standard chemoradiation therapy, hyperfractionated radiotherapy with concurrent chemotherapy, delivered continuously or as a split course, has not been shown to increase survival in randomized studies. However, studies have demonstrated improved outcomes with hyperfractionated accelerated radiotherapy (HART). In one randomized trial, the 2-year survival rate was better with continuous HART, delivering 54 Gy in 36 fractions of 1.5 Gy over 12 days, than with conventional radiotherapy alone, 60 Gy in 30 fractions (29% vs. 20%). In Eastern Cooperative Oncology Group (ECOG) 2597, patients were given two cycles of carboplatin and paclitaxel and then randomly assigned to HART (1.5 Gy three times per day for 2.5 weeks) or standard radiotherapy (64 Gy in 2-Gy daily fractions). There was a nonsignificant improvement in median survival (20.3 months vs. 14.9 months, p = 0.28) and 3-year overall survival (23% vs. 14%) for patients in the HART arm.
The most informative results come from a meta-analysis of data from 2000 patients (eight trials) who had been randomly assigned to an altered regimen or conventional fractionation. The analysis was limited to trials in which the chemotherapy was identical in both treatment arms. Modified fractionation resulted in a small, but significant, improvement in 5-year overall survival (10.8% vs. 8.3%; hazard ratio, 0.88; 95% confidence interval [CI], 0.80 to 0.97; p = 0.009). Severe esophageal toxicity was more frequent in the modified fraction group (19% vs. 9%).
Widespread adoption of modified radiotherapy schedules instead of conventional once-daily treatments has been limited by the logistical challenges of HART for the patient and treatment centers, as well as the higher rates of toxicity.
Hypofractionation
Hypofractionated radiotherapy is the delivery of fewer, larger (>2 Gy) doses of radiotherapy and is another potential strategy for improving dose intensity. This approach has become more feasible as a result of decreasing radiotherapy volumes, which allow for more conformal radiotherapy delivery and limit the dose delivered to normal tissue. Few studies have evaluated hypofractionation with modern radiotherapy techniques for locally advanced NSCLC. Two prospective phase II studies evaluating concurrent platinum-based chemotherapy with radiotherapy (2.4 Gy/d to 2.75 Gy/d) have reported an encouraging median survival of 20 months. In the sequential or concurrent cancer radiation (SOCCAR) trial, 55 Gy was delivered in 20 fractions over 4 weeks with sequential or concurrent chemotherapy (cisplatin [DDP]–vinorelbine). In this limited phase II trial, 2-year survival rates were similar (50% vs. 46%) with 8% experiencing grade 3 esophagitis. Additional studies using modern radiotherapy techniques are currently being conducted within a cooperative group setting as well as in single institutions. One study of interest is a phase III trial comparing a hypofractionated course of 60 Gy in 15 fractions over 3 weeks with conventional radiotherapy (60–66 Gy in 30–33 fractions over 6 weeks to 7 weeks) without concurrent chemotherapy for patients with stage II–III NSCLC and poor performance status (NCT01459497).
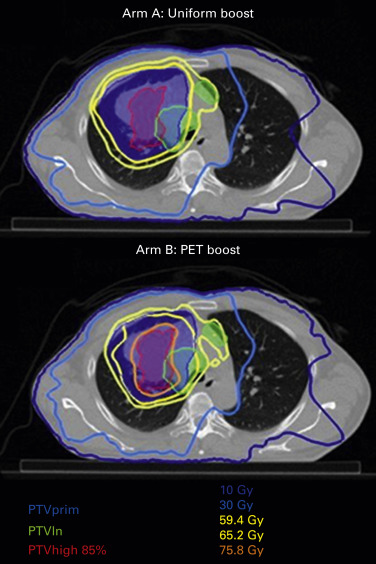
Ongoing research is examining isotoxic dose escalation based on normal tissue tolerance or using the stereotactic body irradiation therapy technique to increase the dose to 18 F-2-deoxy- d -glucose-avid portions of the tumor based on intratreatment PET–CT. Currently, a randomized trial is comparing a homogeneous dose distribution to the primary tumor or a heterogeneous dose distribution based on the metabolic image provided by a PET–CT ( Fig. 39.1 ). Last but not least, protons are under investigation in stage III NSCLC to take advantage of better dose distribution, especially allowing better sparing of the heart, but the results of a randomized trial were disappointing.
In summary, higher physical or biologic dose (altered fractionation) is associated with better local control and, in some trials, with better survival, but the optimal dose and fractionation are yet to be defined. Currently, 60 Gy to 66 Gy in daily fractions of 2 Gy remains the most common schedule.
Chemoradiation Therapy
Chemoradiation therapy is now the standard treatment for stage III NSCLC classified as N2 or N3. Results from meta-analyses of patients with unresectable stage III locally advanced NSCLC have demonstrated the benefits of platinum-based chemoradiation therapy given concurrently or sequentially in comparison with radiation alone. Furthermore, a third meta-analysis has clearly demonstrated the superiority of a concurrent approach to sequential treatment.
Role of Chemotherapy
For patients with medically inoperable or technically unresectable locally advanced NSCLC, thoracic radiotherapy alone, which is potentially curative, was regarded as a standard therapy in the 1980s; however, the treatment results were unsatisfactory due to a high rate of relapse and distant metastases. It was thought that chemotherapy with radiosensitizing anticancer drugs might improve survival by controlling remote metastases and increasing tumor sensitivity to radiotherapy, and several trials tested this hypothesis. Results from meta-analyses showed that survival after sequential or concurrent chemoradiation therapy that included a platinum agent was better than survival after radiotherapy alone. The important role of chemotherapy was demonstrated with an absolute benefit of 3% at 2 years and 2% at 5 years. Furthermore, a third meta-analysis has clearly demonstrated the superiority of a concurrent to a sequential approach.
Nevertheless, these findings were still not satisfactory, and subsequent investigations aimed to establish the optimal timing and type of chemotherapy needed to control micrometastases, increase the effects of radiotherapy, and improve local control and survival.
Sequential and Concurrent Therapy
Sequential chemoradiation therapy has been compared with concurrent chemoradiation therapy in several studies ( Table 39.1 ). The first published trial was from Furuse et al.: radiotherapy (56 Gy using a split-course schedule) was given either concurrently or after an induction with mitomycin, vindesine, and DDP in unresectable locally advanced NSCLC. Median survival was significantly superior in patients receiving concurrent therapy (16.5 months), as compared with those receiving sequential therapy (13.3 months; p = 0.03998). The 5-year survival in the concurrent group (15.8%) was better than that in the sequential group (8.9%). The three other trials showed a trend in favor of the concurrent arm. RTOG 9410 was a randomized three-arm phase III trial comparing sequential with concurrent chemoradiation therapy. The sequential arm consisted of DDP (100 mg/m 2 ) on days 1 and 29 and vinblastine (5 mg/m 2 ) per week for 5 weeks with chest radiotherapy (60 Gy) starting on day 50. One of the concurrent arms used the same chemotherapy regimen as the sequential arm with thoracic radiotherapy (60 Gy) starting on day 1. This concurrent arm had a significantly better 5-year survival rate compared with the sequential arm (16% vs. 10%, p = 0.046).
| Investigators | No. of Patients | RT, Gy | Chemotherapy Regimen | Median Survival Time (Mo) | 2-Year Survival Rate, % | 5-Year Survival Rate, % |
|---|---|---|---|---|---|---|
| Furuse et al. Fournel et al. Zatloukal et al. Curran et al. | 156 158 100 101 52 50 193 200 199 | 56 56 66 66 60 60 69.6 63 63 | Conc RT DDP + MIT + VDS × 2 DDP + MIT + VDS × 2 → Seq RT Conc RT DDP + ETP × 2 DDP + VNR × 2 DDP + VNR × 3 → Seq RT DDP + VNR → Conc RT DDP + VNR × 2 → DDP + VNR DDP + VNR × 4 → Seq RT Conc RT DDP + ETP × 2 Conc RT DDP + VLB × 2 DDP + VLB × 2 → Seq RT | 16.5 13.3 16.3 14.5 16.6 12.9 15.2 17.0 14.6 | 34.6 27.4 39 26 34.2 14.3 34 35 32 | 15.8 8.9 21 a 14 a 18.6 9.3 13 16 10 |
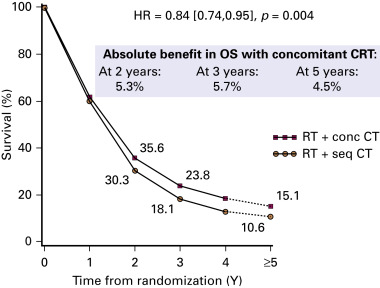
The NSCLC Collaborative Group performed a meta-analysis of six randomized trials and was based on individual patient data. Compared with sequential chemoradiation therapy, concurrent chemoradiation therapy significantly improved overall survival (hazard ratio, 0.84; 95% CI, 0.74 to 0.95; p = 0.004) with an absolute benefit of 5.7% (23.8% vs. 18.1%) at 3 years and 4.5% (15.1% vs. 10.6%) at 5 years ( Fig. 39.2 ). This benefit was mainly due to less locoregional progression without any difference in the rate of distant metastases. Concurrent chemoradiation therapy increased acute esophageal toxicity (grade 3–4) from 4% to 18% with a relative risk of 4.9 (95% CI, 3.1 to 7.8; p < 0.001). There was no significant difference in acute pulmonary toxicity.
In summary, concurrent DDP-based chemoradiation therapy has been consistently shown to improve survival at the cost of manageable increased toxicity. Concurrent chemoradiation therapy that includes DDP is recommended as the standard therapy for patients with inoperable locally advanced NSCLC who are eligible for radiotherapy. Sequential chemoradiation therapy or radiotherapy alone is appropriate for frail patients who are unable to tolerate concurrent chemoradiation therapy.
Chemotherapy Drug Combinations
Several phase III trials of chemoradiation therapy with platinum (especially DDP) and second-generation anticancer agents, such as vindesine, mitomycin, etoposide, and vinblastine, have produced strong evidence of the effectiveness of these drugs, as described earlier. The evidence for combination therapy with platinum and third-generation agents for the treatment of locally advanced NSCLC has been less conclusive, although some trials have shown that such a combination produces significant response and survival when used to treat stage IV NSCLC. Additional data are needed to determine the optimal regimen. Several randomized trials are reviewed in the following sections (also see Tables 39.2 and 39.3 ).
| Investigators | No. of Patients | RT, Gy | Chemotherapy Regimen | Median Survival Time (Mo) | 2-Year Survival, % |
|---|---|---|---|---|---|
| Belani et al. Zatloukal et al. Yamamoto et al. Segawa et al. Wang et al. Senan et al. | 91 74 92 52 50 146 147 147 101 99 33 32 301 297 | 63 63 63 60 60 60S 60 60 60 60 60 66 66 66 | CBDCA + PTX × 2 → Seq RT CBDCA + PTX × 2 → Conc RT CBDCA + PTX × 2 Conc RT CBDCA + PTX × 2 → CBDCA + PTX × 2 DDP + VNR → Conc RT DDP + VNR × 2 → DDP + VNR DDP + VNR × 4 → Seq RT Conc RT DDP + VDS + MIT × 2 DDP + VDS + MIT × 2 Conc RT CBDCA + IRIN × 2 DDP + IRIN × 2 Conc RT CBDCA + PTX × 2 CBDCA + PTX × 2 Conc RT MIT + VDS + DDP × 2 Conc RT DOC + DDP × 2 Conc DDP + ETP × 2 Conc CBDCA + PTX weekly Conc RT DDP + PEM → PEM × 4 Conc RT DDP + ETP → DDP + X × 2 Conc RT DDP + PEM × 4 + Cetux → PEM × 4 | 13 12.7 16.3 16.6 12.9 20.5 19.8 22.0 23.7. 26.8 20.2 13.5 26.8 25 | 30 25 31 34.2 14.3 17.5 17.8 19.5 48.1 60.3 36.4 16.2 52 52 |
| Study | Agent | Study design | Findings |
|---|---|---|---|
| RTOG 0324 Phase II | Cetuximab | Carboplatin/paclitaxel/cetuximab/RT → carboplatin/paclitaxel × 2 cycles | Median OS 27.7 mo; 2-y OS 49.3% |
| CALGB 30407 Phase II | Cetuximab | Carboplatin/pemetrexed/RT ± cetuximab | Without cetuximab: 18 mo; OS 58% With cetuximab: 18 mo; OS 52% |
| SWOG 0023 Phase III | Gefitinib | Chemo/RT → docetaxel × 3 cycles → gefitinib versus placebo | Gefitinib: median OS 23 mo Placebo: median OS 35 mo |
| CALGB 30106 Phase II | Gefitinib | Poor risk group: carboplatin/paclitaxel → RT/gefitinib → gefitinib Good risk group: carboplatin/paclitaxel → RT/gefitinib/carboplatin/paclitaxel → gefitinib | Poor risk group: PFS 13.4 mo, median OS 19 mo Good risk group: PFS 9.2 mo, median OS 13 mo |
| University of Chicago Phase I | Erlotinib | Group 1: carboplatin/paclitaxel → carboplatin/paclitaxel/RT/erlotinib Group 2: cisplatin/etoposide/RT/erlotinib → docetaxel | Group 1: median OS 13.7 mo Group 2: median OS 10.2 mo |
| Spigel et al. Phase II | Bevacizumab | Carboplatin/pemetrexed/bevacizumab/RT → carboplatin/pemetrexed/bevacizumab → bevacizumab | 2/5 patients developed tracheoesophageal fistulae |
| ECOG 3598 Phase III | Thalidomide | Carboplatin/paclitaxel/RT ± thalidomide | 1-y survival, 57% carboplatin/paclitaxel; 67% on the thalidomide arm 2-y survival, 34% and 33% |
| RTOG 0617 Phase III | Cetuximab | Carboplatin/palictaxel/RT ± cetuximab | Median overall survival with cetuximab 23.1 mo; 23.5 mo in those not receiving cetuximab |
Belani et al. enrolled patients with unresectable locally advanced NSCLC in a three-arm phase II trial. Patients in arm 1 (sequential arm) received two cycles of induction chemotherapy with paclitaxel (200 mg/m 2 ) and carboplatin (area under the curve [AUC] = 6) followed by radiotherapy (63 Gy). Patients in arm 2 (induction and concurrent) received two cycles of induction chemotherapy with paclitaxel (200 mg/m 2 ) and carboplatin (AUC = 6) followed by weekly paclitaxel (45 mg/m 2 ) and carboplatin (AUC = 2) with concurrent radiation (63 Gy). Patients in arm 3 (concurrent and consolidation) received weekly paclitaxel (45 mg/m 2 ), carboplatin (AUC = 2), and radiotherapy (63 Gy) followed by two cycles of paclitaxel (200 mg/m 2 ) and carboplatin (AUC = 6). The median overall survival was 13.0 months, 12.7 months, and 16.3 months for arms 1, 2, and 3, respectively. The proportions of survivors in arm 1 at 1 year, 2 years, and 3 years were 57%, 30%, and 17%, respectively; in arm 2, 53%, 25%, and 15%, respectively; and in arm 3, 63%, 31%, and 17%, respectively. In this study, concurrent weekly paclitaxel, carboplatin, and thoracic radiotherapy followed by consolidation was associated with the best outcome but with greater toxicity.
Reduced (lower-dose) DDP and vinorelbine combination therapy is widely used as a standard treatment, but few prospective trials have been conducted. Zatloukal et al. demonstrated the safety and efficacy of this combination in a trial of concurrent and sequential chemoradiation therapy for patients with locally advanced NSCLC. Fifty-two patients were randomly assigned to concurrent treatment and 50 patients to sequential treatment. The chemotherapy consisted of up to four cycles of DDP (80 mg/m 2 ) on day 1, and vinorelbine (25 mg/m 2 in the 1st and 4th cycles; 12.5 mg/m 2 during the 2nd and 3rd cycles) on days 1, 8, and 15, of a 28-day cycle. Radiotherapy (60 Gy) was given as five fractions per week for 6 weeks. In the concurrent arm, radiotherapy began on day 4 of cycle 2; in the sequential arm, it was started 2 weeks after completion of chemotherapy. Overall survival was significantly better in the concurrent arm (median survival, 16.6 months) than in the sequential arm (median survival, 12.9 months; p = 0.023, hazard ratio = 0.61; 95% CI, 0.39–0.93). The proportions of survivors were greater in the concurrent arm than in the sequential treatment arm at years 1, 2, and 3 (69.2%, 34.2%, and 18.6% vs. 53.0%, 14.3%, and 9.5%, respectively). Although the concurrent schedule was associated with a higher toxicity, the adverse event profile was acceptable in both arms.
Two phase III trials comparing second-generation to third-generation chemotherapy in combination with concurrent thoracic radiotherapy were conducted in Japan. The West Japan Oncology Group conducted a three-arm randomized trial comparing a combination of mitomycin, vindesine, and DDP with irinotecan and carboplatin in one experimental arm and paclitaxel and carboplatin in the other. The Okayama Lung Cancer Study Group also used mitomycin, vindesine, and DDP as a control regimen, which they compared with docetaxel and DDP. No difference was noted among the regimens in terms of survival, but febrile neutropenia occurred more often in the control arm.
Lastly, a small trial was conducted in China to compare the DDP and etoposide combination with that of carboplatin and paclitaxel and concurrent radiotherapy (60 Gy). Results from this trial showed that 3-year survival was better after treatment with DDP and etoposide (33% vs. 13%).
Pemetrexed has commonly been used recently in advanced nonsquamous NSCLC with a better outcome. The PROCLAIM study is a phase III trial of pemetrexed and DDP chemotherapy combined with concurrent radiotherapy, followed by consolidation pemetrexed or two additional cycles of a platinum-based regimen. Because pemetrexed can be given in full doses with radical radiotherapy, there was hope that it might result in lower rates of distant metastasis. However, the 2-year survival rates for the two arms were 52% without any significant difference regardless of the end points.
Induction and Consolidation Therapy
Even with concurrent chemoradiation therapy for locally advanced NSCLC, local and distant disease recurrences are a common event, and most patients die of progressive lung cancer. Early administration of full-dose systemic chemotherapy has the potential to improve survival by treating micrometastases early and downstaging the primary tumor before chemoradiation therapy.
A Cancer and Leukemia Group B (CALGB) study randomized 366 patients with stage III NSCLC to immediate chemoradiation therapy (carboplatin, paclitaxel, and 66 Gy of radiotherapy) or induction chemotherapy with two cycles of carboplatin and paclitaxel before chemoradiation therapy. Survival differences were not significant ( p = 0.3), with a median survival of 12 months (95% CI, 10 months to 16 months) and 14 months (95% CI, 11 months to 16 months), respectively. The 2-year survival was 29% (95% CI, 22% to 35%) for immediate chemoradiation therapy and 31% (95% CI, 25% to 38%) for induction chemotherapy. The addition of induction chemotherapy to concurrent chemoradiation therapy added toxicity and provided no survival benefit compared with concurrent chemoradiation therapy alone. Similarly, a meta-analysis of individual patient data from six small randomized phase II trials did not show any difference between induction and adjuvant chemotherapy given before or after definitive chemoradiation therapy. A recent trial randomized patients after concurrent chemoradiotherapy to two additional cycles of oral vinorelbine and DDP or best supportive care alone and failed to show any benefit of additional chemotherapy. The Hoosier Oncology Group randomly assigned patients who had already received treatment with DDP, etoposide, and definitive thoracic radiotherapy to consolidation docetaxel or observation. This trial was terminated early because of increased toxicity during docetaxel administration: 5.5% of patients died as a result of this drug. The median survival was 21.2 months in the docetaxel arm compared with 23.2 months in the observation arm ( p = 0.883).
In summary, induction chemotherapy, adjuvant chemotherapy, and/or maintenance therapy currently are not recommended for patients with unresectable locally advanced NSCLC.
Chemoradiation Therapy for Older Individuals
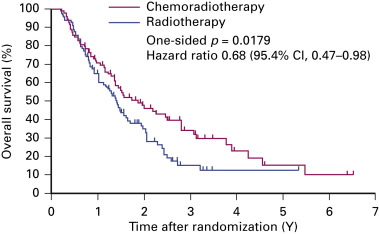
Clinical trials rarely provide data on chemoradiation therapy for individuals older than 70 years. Atagi et al. randomly assigned patients older than 70 with a good performance status to radiotherapy (60 Gy) and concurrent low-dose carboplatin (30 mg/m 2 per day, 5 days a week for 20 days) or to radiotherapy alone. Although greater hematologic toxicity was reported in the combined arm, late toxicities and treatment-related deaths were similar in both arms. Chemoradiation therapy produced a clear survival benefit: the 2-year survival rates were 46% and 35%, respectively ( Fig. 39.3 ). For carefully selected older patients without severe comorbidities, chemoradiation therapy may be considered with careful management of toxicities.
In summary, for most patients with unresectable locally advanced NSCLC, concurrent chemoradiation therapy is the optimal treatment strategy with curative intent. Combination therapy with platinum and second-generation anticancer agents effectively prolongs survival. The superiority or noninferiority of third-generation anticancer drugs has not been shown in phase III trials. Results from smaller studies show that these drugs may modestly increase median survival and 5-year survival. Furthermore, it is difficult to translate the results observed in stage IV to stage III patients treated with a combined approach.
Molecularly Targeted Therapeutic Agents
Recent discoveries in molecular biology have led to the identification of numerous molecular pathways that may be responsible for cancer cell development, progression, and growth; these pathways may also have a role in cancer cell resistance to radiotherapy or other cytotoxic agents. Therefore, these pathways are being explored as potential targets for augmentation of radiotherapy or chemotherapy response. Since the 1990s, there has been an explosion of new molecularly targeted agents for use in lung cancer treatment.
The expanding list of molecular targets for NSCLC includes epidermal growth factor (EGF) and its receptor (EGFR), vascular endothelial growth factor (VEGF) and its receptor (VEGFR), the fusion of echinoderm microtubule-associated protein-like 4 and anaplastic lymphoma kinase ( EML4–ALK ), B-Raf , PIK3CA gene, ErbB2 ( Her2 ) amplification or mutant genes, mammalian target of rapamycin, and various other molecules that regulate different steps in their signal transduction pathways. Although preclinical data would indicate that these molecules are viable targets that could be exploited to improve therapeutic efficacy, not all agents have produced clinical benefits. A handful of targeted agents have been approved for cancer treatment. Clinical trials of other agents are being conducted to determine their efficacy in combination with other cytotoxic agents, including ionizing radiotherapy. Some potential agents target a single molecular signaling pathway, whereas others are able to target multiple molecular signaling pathways. The most clinically advanced agents target the EGFR, VEGF/VEGFR, and ALK1 pathways.
Epidermal Growth Factor Receptor
EGFR targeting exemplifies the approach of combining radiotherapy with molecularly targeted therapy. EGFR plays an important role in tumor growth and response to cytotoxic agents, including ionizing radiotherapy. Upregulation of EGFR expression occurs in many types of cancer and is often associated with more aggressive tumors, poor prognosis, and tumor resistance to treatment with cytotoxic agents including radiotherapy. Preclinical data provide a strong rationale for combining EGFR inhibitors with radiotherapy.
Cetuximab is a chimeric mouse anti-EGFR monoclonal antibody. Although it was a study of patients with head and neck squamous cell carcinoma that confirmed the benefit of cetuximab with radiotherapy, the agent has also been studied extensively in patients with NSCLC. In 2011, CALGB and RTOG reported the results from phase II studies. In the CALGB study, two novel chemotherapy regimens in combination with concurrent radiotherapy were evaluated. Patients in the first group received concurrent carboplatin and pemetrexed with thoracic radiotherapy (70 Gy). In the second group, patients received the same regimen plus cetuximab. Patients in both groups received four cycles of pemetrexed as consolidation therapy. The overall survival at 18 months was 58% without cetuximab and 54% with cetuximab. Treatment of NSCLC with the combination of thoracic radiotherapy, pemetrexed, carboplatin, and with or without cetuximab was shown to be feasible and fairly well tolerated.
In the RTOG study, patients were treated with a combination of paclitaxel, carboplatin, and cetuximab with radiotherapy (63 Gy). All patients received a loading dose of cetuximab (400 mg/m 2 ) 1 week before radiotherapy, and patients received carboplatin, paclitaxel, and cetuximab for two additional cycles after completion of radiotherapy. The median survival was 22.7 months, and the 2-year survival rate was 49.3%. Because of these very promising results, cetuximab was studied in the RTOG 0617 trial in which patients were randomly assigned to receive or not receive cetuximab in addition to concurrent chemoradiotherapy; however, no difference in survival was observed between the two arms. In a separate planned retrospective analysis, the EGFR expression could be evaluated on 203 patients; for patients expressing the EGFR, there was a statistical benefit with a higher survival rate, whereas for those not expressing EGFR, a negative trend was observed. In the Raditux trial, patients received 66 Gy in 24 fractions plus daily DDP with or without cetuximab. The survival rates were very similar but the EGFR expression was not evaluated. More grade 3 lung toxicity was seen in the cetuximab arm (0% vs. 10%).
Gefitinib and erlotinib are two tyrosine kinase inhibitors (TKIs) that are especially active in case of mutation and have been tested with radiotherapy. The Southwest Oncology Group (SWOG) performed a large phase III trial in which patients with stage III NSCLC were treated with standard chemoradiation therapy, and after consolidation with docetaxel for three cycles, the patients were randomly assigned to maintenance therapy with placebo or gefitinib. At interim analysis, overall survival was worse in the gefitinib maintenance arm (23 months vs. 35 months), and therefore the study was closed. It is clear from this study that maintenance therapy with TKI after definitive chemoradiation therapy should be avoided in an unselected patient population.
CALGB 30106 was a phase II study designed to evaluate the addition of gefitinib to sequential or concurrent chemoradiation therapy in patients with unresectable NSCLC. Patients were categorized as poor risk (performance status of 2 or higher, weight loss of 5% or more) or good risk (performance status of 0 or 1, weight loss less than 5%). All patients received induction chemotherapy with two cycles of carboplatin and paclitaxel plus gefitinib. (Gefitinib was removed from induction regimen in May 2004 when the SWOG trial did not demonstrate a benefit to adding gefitinib to chemotherapy.) Patients in the poor risk group received thoracic radiotherapy (66 Gy) with concurrent gefitinib. Patients in the good risk group received the same radiotherapy and gefitinib, but also received weekly carboplatin and paclitaxel. Consolidation gefitinib was given until disease progression. In the poor risk group, progression-free survival was 13.4 months, and median survival was 19 months. In the good risk group, progression-free survival was 9.2 months, and median survival was 13 months. As many as 13 of 45 tumors had activating EGFR mutations, and 2 of 13 had T790M mutations. Seven of 45 tumors had KRAS mutations. When the results were analyzed by these molecular phenotypes, no significant difference in outcome was noted. Given the promising results for patients at poor risk, further studies will investigate the effectiveness of treating such patients with radiotherapy and gefitinib after induction chemotherapy; however, this regimen may not be beneficial for patients at good risk.
The findings from CALGB 30106 are consistent with studies of erlotinib and chemoradiation. Choong et al. reported on a phase I study of erlotinib with chemoradiation. Patients in one group received induction chemotherapy with carboplatin and paclitaxel followed by carboplatin, paclitaxel, radiotherapy, and erlotinib. The second group of patients received DDP, etoposide, radiotherapy, and erlotinib followed by docetaxel. In both arms, the erlotinib dose was increased from 50 mg to 150 mg in three stages. The median survival in each group was 13.7 months and 10.2 months, respectively. Overall survival and progression-free survival were improved for patients in whom a rash developed. This study demonstrated the tolerability of such a regimen, but given the disappointing survival data, the need for improved patient selection criteria for EGFR-based treatments is clear.
Patient selection is likely to play an important role in the design of future studies of EGFR-targeted agents. For example, these agents have shown benefits for patients with EGFR mutations and studies may need to separate patients with activating EGFR mutations from patients with the general wild-type EGFR. A repeat evaluation of biomarkers after chemoradiation therapy may help to determine which subset of patients would benefit from additional therapy with anti-EGFR agents. Therefore molecular profiling, along with patient selection based on criteria such as EGFR mutation status, has become an important factor in predicting efficacy of anti-EGFR regimens. Future studies of anti-EGFR treatments used in combination with radiotherapy should also incorporate stringent patient selection criteria to maximize treatment benefit. The efficacy of combined treatment with EGFR inhibitors and radiotherapy may vary by tumor type and molecular profile, as well as the sequencing of the treatments.
Antiangiogenesis Agents
Inhibitors of angiogenesis have undergone extensive preclinical testing and some agents have been tested in clinical trials. Despite concerns that an antiangiogenic agent would enhance hypoxia, thereby impairing the efficacy of radiotherapy, the first preclinical study with a specific inhibitor of angiogenesis, angiostatin, showed a synergistic effect with radiotherapy. Jain proposed a model of tumor vasculature normalization that explains this effect. In this model, proangiogenic factors from tumors can cause abnormal neovascularization, and inhibition of tumor angiogenesis transiently normalizes the tumor vasculature. This has the counterintuitive effect of decreasing tumor hypoxia and improving the effectiveness of radiotherapy. Preclinical studies support this hypothesis, as have results from a phase I study with locally advanced rectal cancer.
Similar to the EGFR inhibitors, antiangiogenic compounds can be broadly classified as monoclonal antibodies directed against antiangiogenic molecules or their receptors (e.g., bevacizumab) or TKIs with narrow or broad-spectrum activity against one or more of these receptors (e.g., sorafenib, sunitinib, pazopanib). Studies of bevacizumab for the treatment of NSCLC have also included radiotherapy.
Efforts to improve the therapeutic ratio by adding bevacizumab to chemoradiation therapy have failed in multiple studies of patients with both small cell lung cancer (SCLC) and NSCLC. This regimen was associated with increased incidence of tracheoesophageal fistula in both SCLC and NSCLC. Therefore patient selection factors such as location of the tumor and tumor histology, as well as the timing of bevacizumab integration with radiotherapy, need to be considered in the design of future studies.
Thalidomide has also been found to have potent immunomodulatory effects and antiangiogenic properties. ECOG 3598 was a randomized study comparing chemoradiation therapy with or without thalidomide for patients with locally advanced NSCLC. Patients received carboplatin and paclitaxel, with or without thalidomide for two cycles, followed by weekly carboplatin and paclitaxel with radiotherapy, with or without thalidomide. In the thalidomide group, patients could be treated with adjuvant thalidomide for up to 2 years. There was no difference in progression-free survival or overall survival with addition of thalidomide. Although this outcome might suggest that this treatment combination is not effective, the negative studies may also indicate the need for better patient selection when using specific agents.
Anaplastic Lymphoma Kinase Inhibitors
The EML–ALK fusion oncogene has become a very important potential biomarker for patients with NSCLC. Several ALK inhibitors have been identified; crizotinib is the most developed and has produced impressive responses. However, data have not indicated that ALK inhibitors have a radiosensitizing or synergistic effect when administered concurrently in combination with radiotherapy
Immune Checkpoint Inhibitors (PD-1/PD-L-1)
Interesting observations were made with the use of nivolumab and ipilimumab in stage IV patients or in experimental work with an abscopal response after radiotherapy using stereotactic radiotherapy to a metastatic site. Currently, phase III trials are ongoing to evaluate the safety (the concern is risk of radiation pneumonitis) and the efficacy.
In summary, as of 2016, no trial has proven the benefits of adding a molecularly targeted agent to the combined modality regimens in an unselected patient population. Currently, studies adding erlotinib or crizotinib for treatment of patients who are known to have EGFR mutation or ALK translocation are underway by NRG/Alliance in the United States.
The discovery of new biomarkers, advances in molecular therapeutics and imaging technology, and a better understanding of effective integration of chemotherapy and radiation treatments are making personalized medicine for NSCLC feasible. As more precise biomarkers are identified, the use of such personalized strategies will become routine. In addition, immunomodulatory therapy may play a larger role in the treatment of NSCLC. Initial studies of immunomodulatory agents in combination with cytotoxic agents have provided promising early results. Incorporation of such agents into a concurrent chemoradiation therapy is being investigated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree