Fig. 11.1
Patient positioning and immobilization. External radiation treatment is generally performed with the patient in a supine position and wearing a custom-made thermoplastic mask
11.3.1.2 Target Volume
The gross tumor volume (GTV) encompasses the primary tumor and lymphadenopathy. The GTV is determined clinically by computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET). Basically, the clinical target volume (CTV) encompasses the GTV along with a 5–10-mm margin. The prophylactic irradiation range of oral cancer (CTV prophylactic) includes the regional lymph nodes (levels I–III). In cases with cervical lymph node metastasis above stage N2b, levels I–IV nodes are also included in the CTV prophylactic.
Regarding carcinoma of the tongue, the floor of mouth, and other advanced lesions (beyond the midline) or cases with cervical lymph node metastasis, prophylactic irradiation of both sides is required. In well-lateralized buccal mucosal lesions, the upper alveolar ridge and mandibular gingiva are treated by ipsilateral neck irradiation [18]. Well-lateralized tumors of the hard palate without lymph node metastases and bone invasion are low risk, and therefore only the primary tumor should be treated.
The planning target volume (PTV) includes a 5–10-mm margin around CTV. The organs at risk (OAR) include the spinal cord, mandible, and parotid glands.
11.3.1.3 Irradiation Method
Carcinoma of the oral cavity has traditionally been treated with opposed lateral fields, using CT-based two- or three-dimensional techniques. Beams of 4–6 MV are most suitable for the treatment of oral cavity cancers. For patients with N0-stage neck involvement and well-lateralized primary lesions, 40–50 Gy to the ipsilateral neck should be considered as elective nodal irradiation (Figs. 11.2 and 11.3). Mixed-beam or angled-wedge techniques are used depending on the tumor location. In cases with patients with N+neck staging, the dose to the involved lymph nodes should range from 66 Gy to 70 Gy over 6–7 weeks. Patients with advanced lesions and high-risk disease (multiple positive nodes) should receive external beam radiotherapy to the bilateral neck (Fig. 11.4). An additional boost that encompasses the tumor and palpable nodes with appropriate margins should be delivered to avoid a high dose to the spinal cord. For radical radiotherapy, the total dose should be 60–70 Gy over 6–7 weeks.
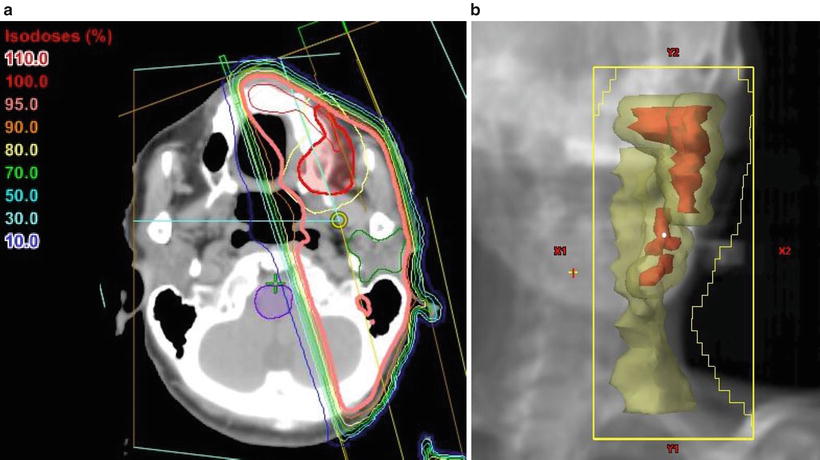
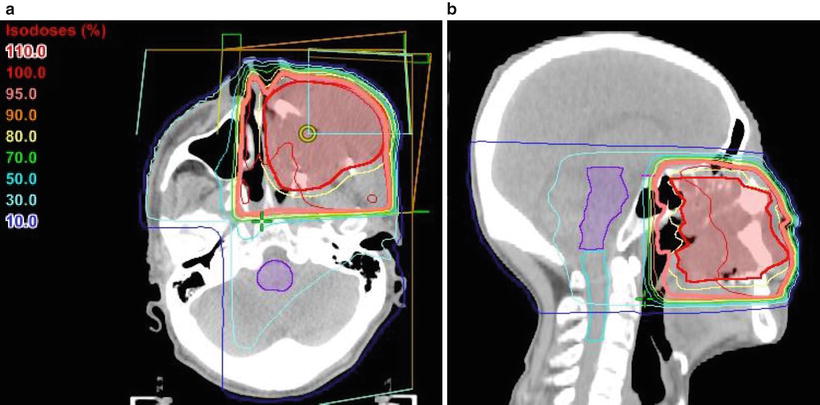
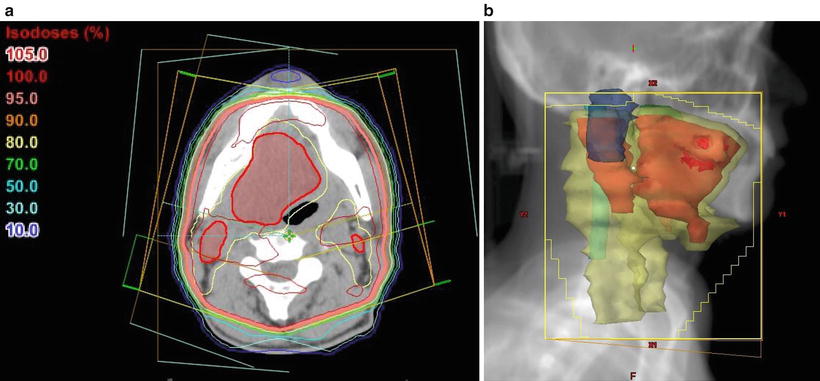

Fig. 11.2
Treatment plan for hemi-neck irradiation. Typical example of the irradiation fields for a carcinoma of the left upper gingiva and buccal mucosa (cT4aN1M0, SCC). The patient was treated ipsilaterally with parallel opposed oblique fields to avoid the spinal cord using CT-based three-dimensional techniques. With a spacer, the tongue could be excluded from the radiation field. (a) CT (axial view); showing the isodose lines, the thick line indicates the 95 % prescribed dose. (b) Beam’s eye view, 340°. Red, tumor [gross tumor volume (GTV)]; yellow, clinical target volume (CTV) plus lymph node region; purple, brain stem

Fig. 11.3
Treatment plan incorporating the wedged-pair technique for anterior lesions of the maxillary gingiva. An example of the irradiation fields for a locally advanced carcinoma of the left upper gingiva (cT4aN0M0, SCC). The patient was vertically irradiated with two portals using angle wedges. Showing the isodose lines, the thick lines indicate the 95 % prescribed dose. (a) CT (axial view). (b) CT (sagittal view)

Fig. 11.4
Treatment plan for whole neck irradiation. An example of the irradiation fields for a carcinoma of the right tongue (cT4bN2cM0, SCC). The patient was treated with lateral opposed photon fields using a wedged-pair technique and “field-in-field” compensation. Two wedged beams at 150° were irradiated from the anterior oblique to avoid the shoulder. The primary site and neck levels I–IV were encompassed by the radiation field. (a) CT (axial view); showing the isodose lines, the thick line indicates the 95 % prescribed dose. (b) Beam’s eye view, 285°. Red, GTV; yellow, CTV; light blue, spinal cord; blue, right parotid gland
When external beam radiotherapy is used as the sole treatment modality, even small lesions require 66 Gy given in 33 fractions for reliable control. For large tumors, more than 70 Gy is required to achieve a good result, but there are increasingly significant consequences regarding normal tissue toxicity with doses in this range.
11.3.1.4 Postoperative Radiotherapy
There is no evidence of clinical significance regarding preoperative irradiation when used in conjunction with surgery; however, postoperative irradiation is common. Postoperative radiotherapy is required for advanced lesions (large primary tumors, bone involvement, extensive perineural, or vascular invasion), positive or close surgical margins, and the presence of multiple positive lymph nodes or ECE [7, 17]. In postoperative cases, the range of irradiation is determined by the pathological results of surgery.
In general, the radiation setup is the same as that for primary radiotherapy, but should be tailored individually depending on the location of the primary lesion. The radiation field should encompass the entire surgical bed and upper neck nodes. In most cases, it is necessary to treat the patient with opposed lateral fields to cover the tumor bed and remaining tumors with appropriate surrounding margins (Fig. 11.5).
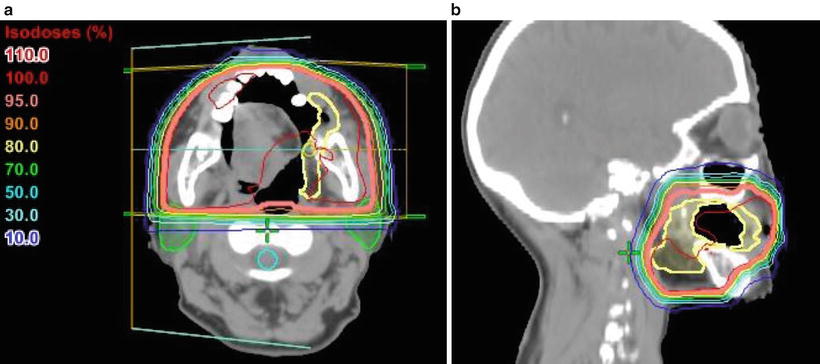

Fig. 11.5
Treatment plan for opposed lateral fields. An example of postoperative irradiation for a carcinoma of the buccal mucosa (cT2N0M0, SCC). The patient underwent partial resection and was treated with postoperative radiotherapy. The margin was free of tumor, and only the primary tumor was irradiated using parallel opposed lateral photon fields to avoid the spinal cord and parotid gland. A total dose of 50 Gy was delivered in 25 fractions. Showing the isodose lines, the thick line indicates the 95 % prescribed dose. (a) CT (axial view). (b) CT (sagittal view). Yellow, CTV; light blue, spinal cord; green, parotid glands
In cases with N0 neck staging, the regional lymph node (levels I–III) is irradiated. In the presence of multiple positive lymph nodes (more than 3) or ECE, the range from levels I to IV should be irradiated. If possible, to improve the quality of life of patients after treatment, dose reductions to the salivary glands and lower jaw are desirable.
If there is no gross residual tumor, the recommended dose is 40–50 Gy over 4–5 weeks. High-risk areas (positive primary surgical bed, close margins, ECE, perineural invasion) should receive an additional boost of up to 60–66 Gy over 6–6.5 weeks. In other immediate-risk areas, 56–60 Gy over 5.5–6 weeks should be administered.
It is ideal to start postoperative radiotherapy as soon as possible after surgical wound healing (usually 1 month after surgery) to reduce the potential risk of prolonged cumulative treatment time.
11.3.1.5 Intensity-Modulated Radiation Therapy
IMRT is an advanced three-dimensional conformal treatment that uses a nonuniform beam intensity pattern with computer-aided optimization to achieve a superior dose distribution. The biggest advantage of IMRT is the ability to produce much higher dose distribution conformities than those achievable with conventional 3D conformal radiation therapy using uniform beam intensities. In particular, IMRT can produce concave-shaped isodose distributions that can more closely follow the shape or boundaries of the target and critical structures in three dimensions. The target volumes to receive various dosage levels are delineated, and the dosimetric plans are generated by inverse planning.
Figure 11.6 shows an example of treatment plan using IMRT for the oral cavity cancer. IMRT can reduce normal tissue toxicities including damage to the spinal cord, major salivary glands, and the mandible [19]. In the treatment of oral cavity cancer, salivary gland disorder becomes a problem. Figure 11.7 shows the difference in dose distribution between conventional radiotherapy and IMRT. In patients with bilateral neck disease, it may be difficult to effectively spare the parotid glands through conventional radiation therapy, particularly when the superior level II cervical lymph nodes are involved. Limiting the mean parotid dose to <25–30 Gy is associated with improved post-radiation salivary function [20]. IMRT is the preferred bilateral neck therapy for oral cancer for minimizing xerostomia. In addition, in oral cavity cancer, treatment with IMRT has contributed to improved survival rates of oral cavity cancer [21]; it is expected to be an excellent therapy.
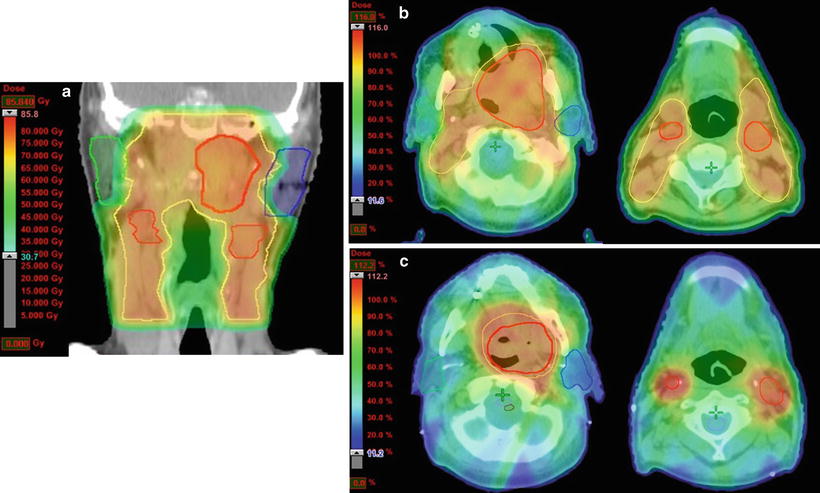
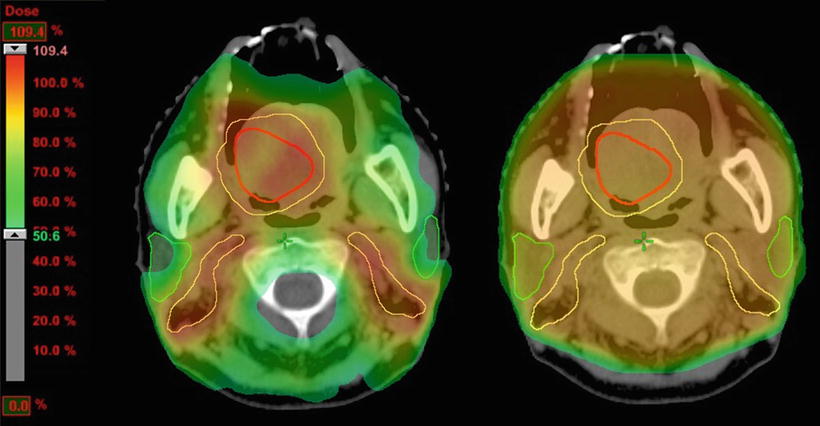

Fig. 11.6
Treatment plan for intensity-modulated radiation therapy (IMRT). An example of the irradiation fields for a carcinoma of the soft palate (cT4aN2cM0, SCC) that was treated with IMRT at a dose of 70 Gy in 35 fractions. (a) Total IMRT dose distribution using a 6-MV photon beam (coronal view). The patient was treated with nine portals to spare the spinal cord and parotid glands. (b) Dose distribution of the first plan using a dose color wash (axial view). The primary tumor and regional lymph node (levels I–III) with margin received 46 Gy in 23 fractions. (c) Dose distribution of the shrinking plan (axial view). The primary tumor and bilateral lymph nodes with 0.5–1-cm margins received a concomitant boost of 24 Gy in 12 fractions for a cumulative prescribed dose of 70 Gy. Red, GTV; yellow, CTV; green, right parotid gland; blue, left parotid gland

Fig. 11.7
Comparison of the dose distributions between IMRT and conventional radiotherapy. An example of the irradiation fields for a carcinoma of the right tongue (cT4aN2bM0, SCC). An image of the photon beam IMRT treatment plan is on the left, and the conventional radiation treatment plan is on the right. IMRT was successfully used to deliver the dose to the CTV and spare the organs at risk. Red, GTV; yellow, CTV; green, parotid glands
11.3.1.6 Electron Beam Therapy (Intraoral Cone Therapy)
Intraoral cone therapy is another option to enable radiation boosting and can be used in place of interstitial radioisotope implants or external radiation therapy for oral cancer.
The tumor must be located in a site accessible to cone placement. The tumor size should not exceed 3 cm at the greatest dimension. No deep invasion of the underlying tissues and no regional or distant metastasis should be present. This technique is suited for anterior oral cavity lesions in edentulous patients and well-differentiated, superficial lesions of the tongue, buccal mucosa, and oral lips. An example of intraoral cone therapy for a carcinoma of the tongue is shown in Fig. 11.8. Electron beam therapy is also effective for cervical lymph node metastases (Fig. 11.9).
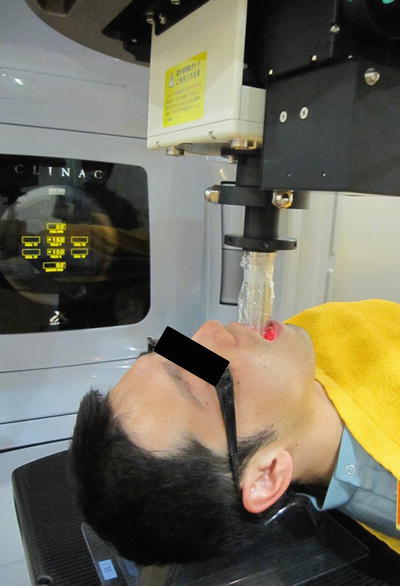
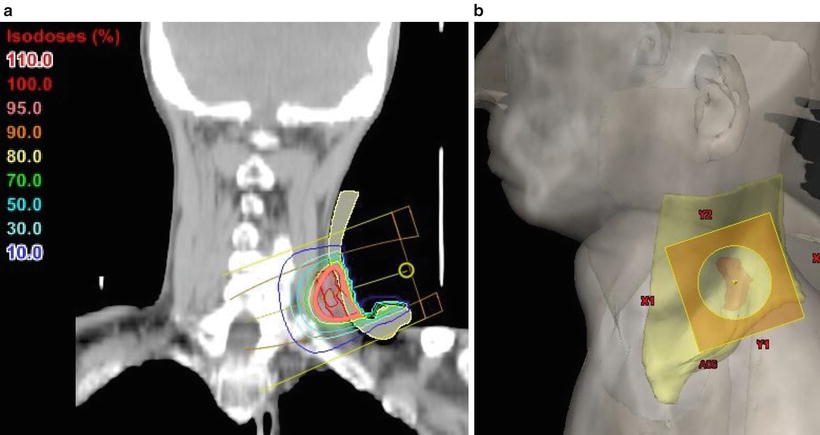

Fig. 11.8
Intraoral cone therapy for a carcinoma of the tongue

Fig. 11.9
A case of electron beam therapy for neck lymph node metastasis. During electron beam therapy, the use of an adequate bolus can increase the surface dose and attenuate the beam to protect underlying tissues. A 6- or 9-MeV electrons are often used. (a) CT (coronal view); showing the isodose lines, the thick line indicates the 95 % prescribed dose. (b) Digital reconstructed radiograph (lateral). Red, tumor (GTV); yellow, bolus; orange, lead plate
The tumor should be tattooed before treatment. The target volume is the primary tumor with a 15–20-mm margin. The intraoral cone remains in constant contact with the oral mucosa during the treatment. Intraoral cone treatment involves electron beams in the 6–12-MeV range. The total dose is 60–70 Gy in 30–35 fractions when intraoral cone therapy is used alone. The boost doses vary between 20 and 30 Gy after a 40–50 Gy external beam radiotherapy course to the primary site and lymph node [22, 23].
Adverse events such as tongue ulcers have been reported with small-fractionated irradiation. However, it is possible to reduce side effects by inserting a lead plate between the tumor and the oral mucosa (such as the buccal mucosa, lips, gums, and tongue) during treatment. The 5-year LC rates and overall survival rates for all patients (T1-T3N0) were reported to be 52 % and 69 %, respectively [24].
11.3.2 Brachytherapy
Brachytherapy represents an effective treatment option and has played an important role in the treatment of oral cavity cancers. This irradiation only affects very localized areas around the radiation sources. Normal tissue exposure to radiation decreases with increasing distance away from the radiation source.
Brachytherapy has been used as a sole treatment modality for superficial early-stage tumors of the oral cavity, with good results. It has also been used to boost primary lesions treated with external beam radiation and can be used in combination with surgery or chemotherapy [25].
Regarding interstitial brachytherapy, the radioactive sources are placed directly in the target tissue. There are two main types of treatment, according to the level or intensity. Low-dose-rate (LDR) brachytherapy involves implanting radiation sources that emit radiation at a rate of up to 2 Gy/h. High-dose rate (HDR) brachytherapy is used when the dose delivery rate exceeds 12 Gy/h.
The placement of radiation sources in the target area can be temporary or permanent. Temporary brachytherapy involves the placement of radiation sources for a set duration before withdrawal. Permanent brachytherapy, also known as seed implantation, involves the placement of small LDR radioactive sources in the tumor or treatment site, which are left permanently to gradually decay.
11.3.2.1 LDR Treatment Method
192Ir wires or single pins, 137Cs needles, and 198Au seeds have been used as radiation sources (Fig. 11.10). The target volume should encompass the tumor with a margin of at least 5 mm. Radiation sources are arranged to cover the PTV, and dose calculations are performed with a dedicated treatment planning system [26]. Particularly for temporary implants, the treatment duration and removal date and time are determined by these calculations.
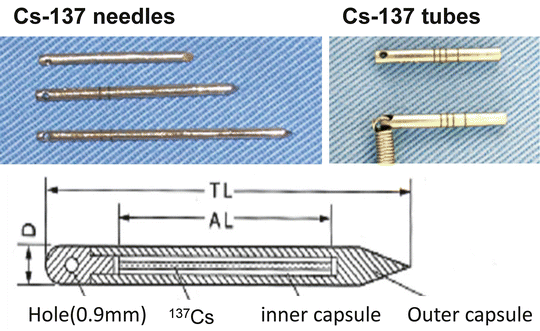

Fig. 11.10
137Cs radiation sources. 137Cs seeds range from 1.5 to 4.5 cm in length. The γ-ray energy is 0.662 MeV, and the half-life is approximately 30.17 years (With permission from Okimoto T and Nishio M, Department of Radiology, Hokkaido Cancer Center)
Radiation sources are usually implanted under local anesthesia. Depending on the lesion size, a single plane, double plane, or volume implant may be selected to cover the tumor with a 5-mm margin. Brachytherapy is usually performed for lesions less than 1 cm thick. For tumors of <1 cm thick, single-plane implants are adequate (Fig. 11.11). However, for tumors of <2 cm thick, double-plane implants are performed (Fig. 11.12). Further, for tumors of <2.5 cm thick, three-dimensional implantation may be possible (Fig. 11.13) [27].
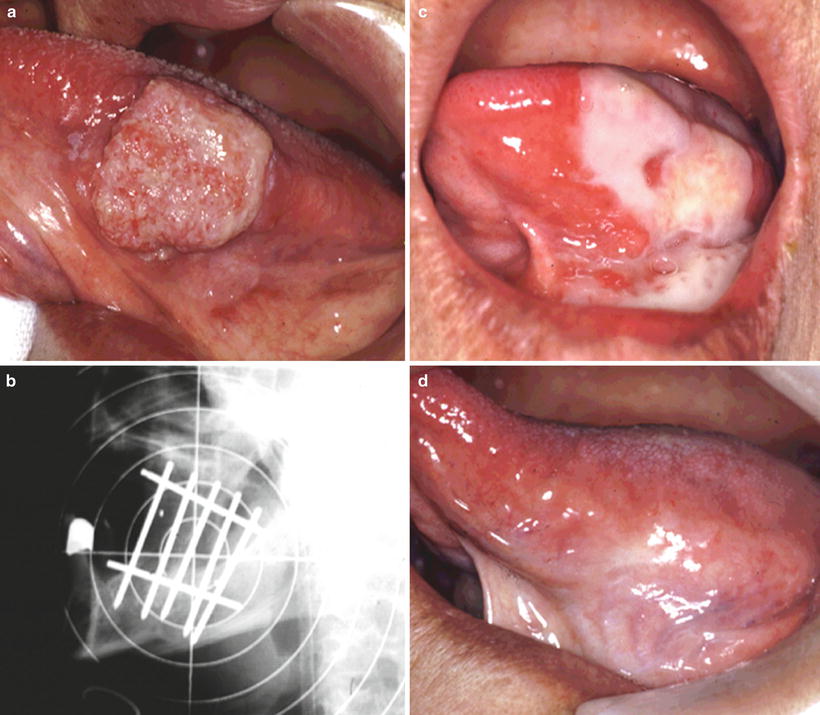
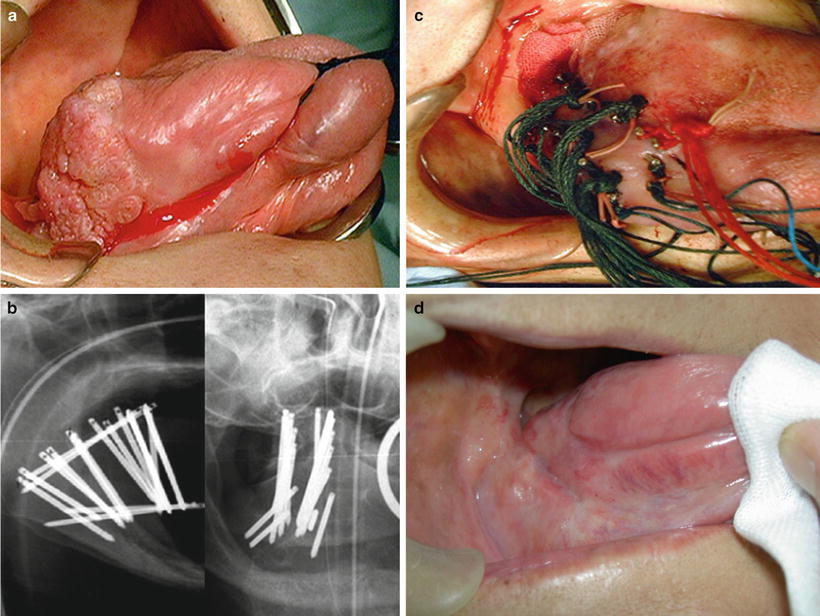
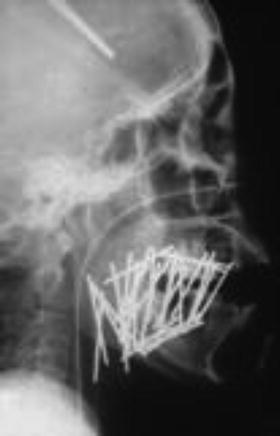

Fig. 11.11
Single-plane implant using 137Cs needles. An example of low-dose-rate (LDR) brachytherapy with a 137Cs source for a carcinoma of the left tongue (cT2N0M0, SCC). (a) Before treatment. (b) Single-plane implant (lateral X-ray). (c) Mucositis after treatment; the range of mucositis is limited to this area. (d) 5 years after treatment (With permission from Okimoto T and Nishio M, Department of Radiology, Hokkaido Cancer Center)

Fig. 11.12
Double-plane implant using 137Cs needles and tumor-volume reduction brachytherapy. An example of LDR brachytherapy with a 137Cs source for carcinoma of the right tongue (cT3N0M0). Before needle insertion, the tumor is trimmed with a laser to reduce the volume. This treatment aims to reduce the incidence of adverse events and improve the dose distribution. (a) Before treatment. (b) Double-plane implant. (c) Radiographs of 137Cs implant. (d) 5 years after treatment (With permission from Okimoto T and Nishio M, Department of Radiology, Hokkaido Cancer Center)

Fig. 11.13
Lateral X-ray of a volume implant using 137Cs needles. An example of three-dimensional implantation of a 137Cs source for a thick lesion
Though the tongue mobility is limited by the rigidity of the cesium needles, 198Au seeds are so small (2.5 × 0.8 mm) that patients can eat and speak during treatment. Figure 11.14 shows 198Au seed implantation for tumor of the floor of the mouth. 198Au seeds can be used for older patients with complications, but variations in dose distributions are likely to occur relative to the other two radiation sources. Therefore, it is used for superficial small lesions for the uniformity of the dose distribution [25, 28].
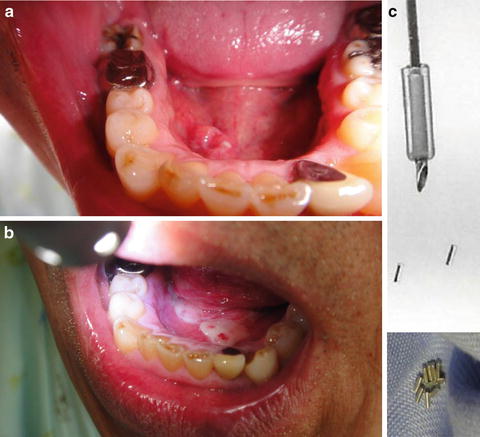

Fig. 11.14
Permanent implant using 198Au seeds. An example of LDR brachytherapy with198Au seeds for a carcinoma of the floor of the mouth (cT2N0M0, 74 Gy/150 MBq, 15 seeds). 198Au seeds are 2.5 × 0.8 mm in size. The γ-ray energy is 0.41 MeV, and the half-life is 2.7 days. (a) Before treatment. (b) 2 weeks after treatment; mucositis is locally observed. (c) 198Au seeds and inserter (holder) (With permission from Okimoto T and Nishio M, Department of Radiology, Hokkaido Cancer Center)
As a sole treatment modality, 192Ir or 137Cs temporary implants and 198Au seeds permanent implants can be used to deliver 65–70 Gy in 5–7 days. In combination with 30 Gy of external beam therapy, doses of 50–70 Gy are often prescribed.
To reduce the risk of ORN, a custom-made plastic device is placed between the tongue and gum, which increase the distance between the radioactive sources and alveolar structures and decrease the radiation exposure to the normal tissues [25]. The risk of adverse effects is increased when combined with external beam radiation. Because LDR irradiation has a lower oxygen enhancement ratio (OER) and reoxygenation of the tumor cell occurs during treatment, the therapeutic ratio is high, and it has desirable properties. However, problems associated with LDR include isolation in the treatment room within a radiation-controlled area and exposure of the medical staff.
11.3.2.2 HDR Treatment Method
HDR brachytherapy is performed through a remote afterloading system (RALS) using an 192Ir or cobalt (60Co) microradiation source. First, guide needles are inserted from the submandibular region either freehand or with the aid of a custom template to facilitate optimal spacing. Applicators are then positioned in their places. Subsequently, the guide needles are removed and buttons were fastened on the skin side. Dose calculations are performed with a dedicated treatment planning system. The dose evaluation is defined as a plane 5 mm from the central applicator. Radiation sources are afterloaded using the tubes. Figure 11.15 shows an example of HDR interstitial brachytherapy using 192Ir.
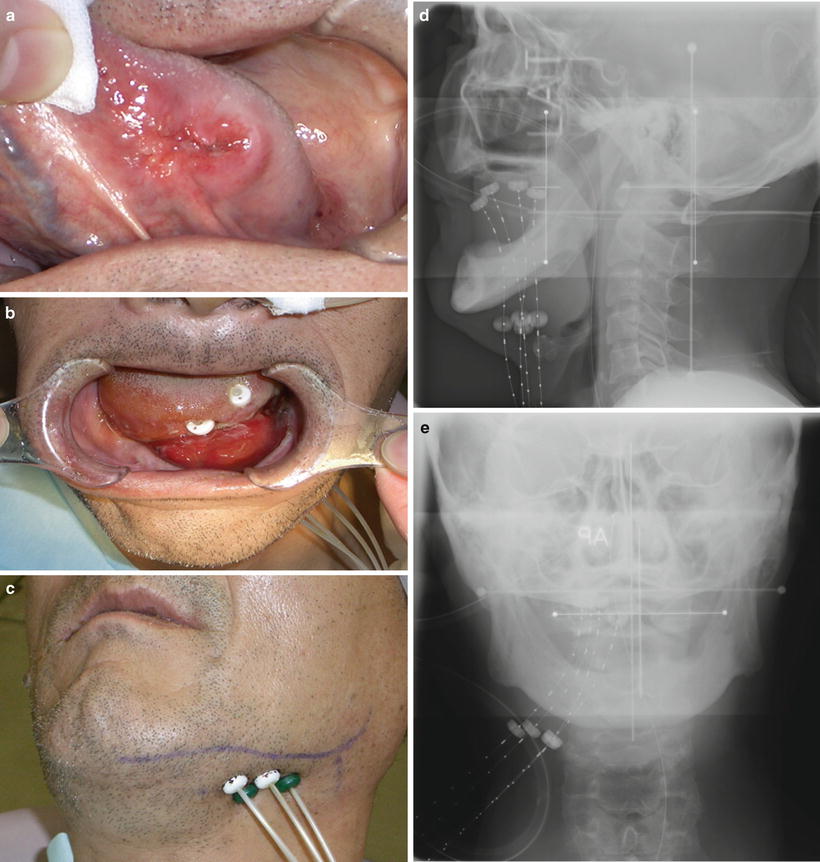

Fig. 11.15
High-dose-rate (HDR) brachytherapy through a remote afterloading system (RALS). An example of HDR interstitial brachytherapy with an 192Ir source for a carcinoma of the left tongue (cT2N0M0). (a) Before treatment. (b, c) Using a submental approach, guide needles were inserted into the anterior floor of the mouth and oral tongue, and hollow tubes (applicators with buttons) were then positioned in their places. Subsequently, the guide needles were removed and buttons were fastened on the skin side. The 192Ir radiation sources were afterloaded using the tubes. (d) Radiograph (lateral port). (e) Radiograph (anterior-posterior port)
There is no need for an isolated treatment room and no risk of medical staff exposure, and it is easy to adjust the prescribed dose. However, the risk of ORN is reportedly higher with HDR than with LDR [29]. Fractionated irradiation is performed at doses of 10–12 Gy, given in 2 fractions per day, for a total dose of 55–60 Gy (9–10 fractions) in 5–7 days. The reported 5-year LC rates were 92.9 %, 81.9 %, and 71.8 % for T1, T2a, and T2b disease [2]. Other good therapeutic results with HDR have also been reported [30].
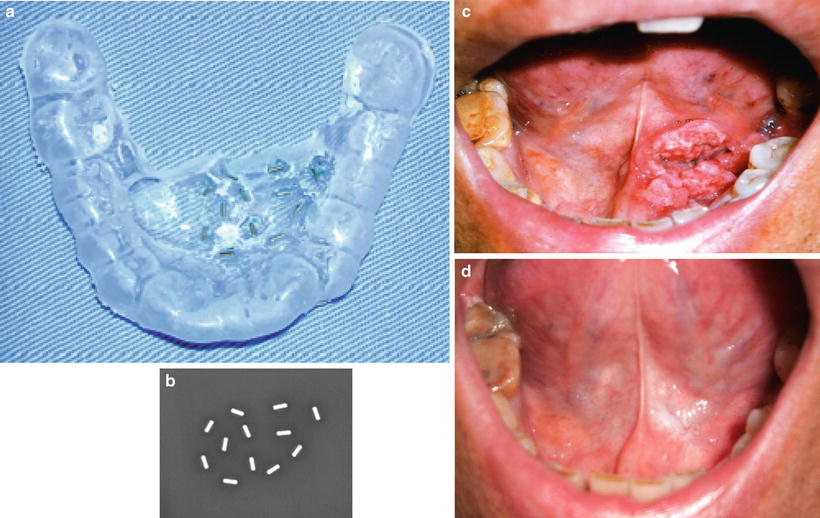
11.3.2.3 Mold Therapy
Although an interstitial implantation is difficult for lesions of the hard palate and alveolar ridge, surface mold radiation can also be considered for small superficial tumors of the lip, hard palate, lower gingiva, and floor of the mouth.
Brachytherapy with a customized intraoral mold is also effective for superficial oral cavity cancers. In order to reduce the doses to the normal tissues, a spacer with lead plates is effective. For radioactive sources, 192Ir is used for HDR and 198Au is usually used for LDR. Figure 11.16 shows an example of HDR blachytherapy with mold therapy using 198Au, and an example of mold device is shown in Fig. 11.17.