During radiotherapy
After radiotherapy
Mucositis
Salivary gland dysfunction
Taste disturbance
Caries
Periodontal disease
Salivary gland dysfunction
Trismus
Osteoradionecrosis of jaws
Hypoplasia of jaws
Dental developmental abnormalities
15.1.1.1 Radiation-Induced Oral Mucositis (Figs. 15.1 and 15.2)
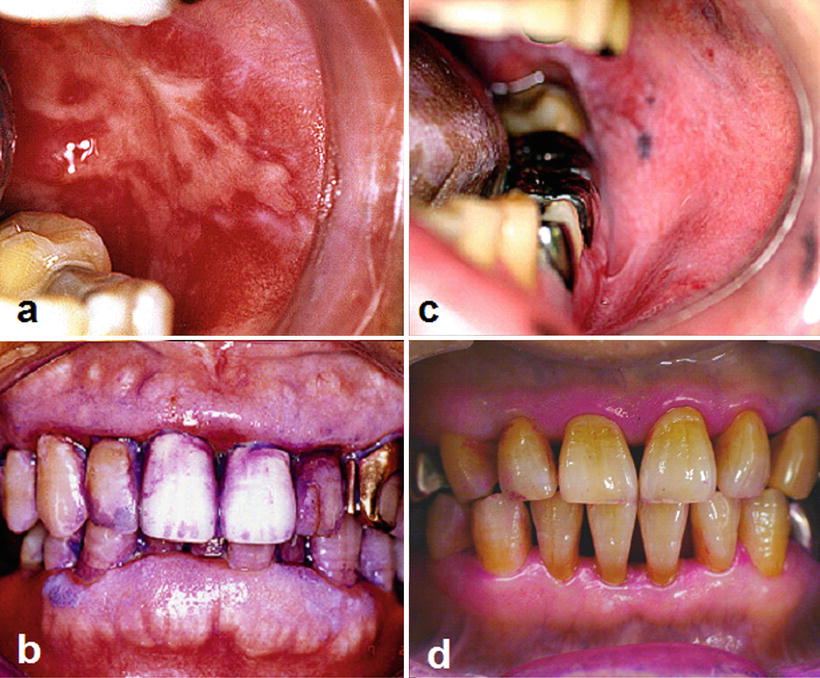
Fig. 15.1
Radiation-induced oral mucositis. Patients with tongue cancer receiving external beam radiotherapy with concurrent chemotherapy (radiation dose in the oral cavity: 56 Gy). Intraoral photographs indicate that the patient with poor oral hygiene (a, b) suffered from more severe oral mucositis compared with the patient with good oral hygiene (c, d) despite receiving the same radiation dose
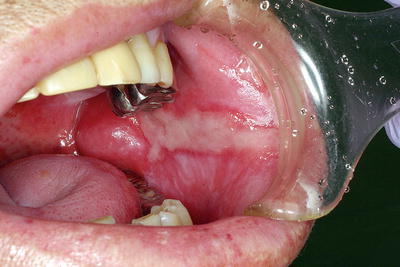
Fig. 15.2
Radiation-induced oral mucositis. A patient with nasopharyngeal cancer who received intensity-modulated radiation therapy (IMRT). Intraoral photograph at 36 Gy indicates an exaggerated mucositis along the dental arch by backscattered radiation
Radiation-induced oral mucositis is a frequent and potentially dose-limiting acute adverse event that afflicts most radiation patients. Severe oral mucositis of grade 3 or 4 according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0 occurs in 35 % and 45 % of head and neck cancer patients undergoing radiotherapy alone and concurrent chemoradiotherapy, respectively. Radiation-induced oral mucositis directly affects patient prognosis given that half of the patients with such severe mucositis require a change in treatment regimen and/or a decrease in the total radiation dose. In addition, radiation-induced mucositis of the head and neck region may not only lead to pain but also to aspiration pneumonia and malnutrition due to oral and pharyngeal dysfunction. The common sites where oral mucositis occurs are the buccal mucosa, tongue margin, and soft palate, although mucositis occurs on all mucosa included within the radiation field. The pathway of radiation- and chemotherapy-induced mucositis is described by Sonis [1]. Clinically, it occurs from day 7 of irradiation and becomes worse in a dose-dependent manner; it heals promptly within 2–3 weeks after the end of radiotherapy provided that there are no accompanying bacterial infections [1, 2].
The risk factors of radiation-induced oral mucositis are shown in Table 15.2. Accelerated hyperfractionation and concurrent chemotherapy are the most important treatment factors for oral mucositis. Since most of the patient-related factors of mucositis can be avoided by oral and dental management, such management before and during radiotherapy is of paramount importance. Recently, various clinical trials using sucralfate, chlorhexidine, or antimicrobial lozenges have been performed to prevent and treat radiation-induced oral mucositis; however, the Multinational Association of Supportive Care in Cancer (MASCC) clinical practice guidelines recommend oral care, midline radiation blocks, three-dimensional radiation, and benzydamine [3].
Table 15.2
Risk factors for radiation-induced oral mucositis
Patient factors | Treatment factors |
|---|---|
Age Gender Europhile count Nutritional status Tobacco Alcohol Oral hygiene Amount of saliva Orthodontic appliance Dental metal Sharp tooth edge | Total radiation dose Radiation dose rate Radiation fraction size Radiation field size Radiation site Concurrent chemotherapy |
15.1.1.2 Radiation-Induced Salivary Gland Dysfunction (Radiation Xerostomia) and Taste Disturbance (Fig. 15.3)
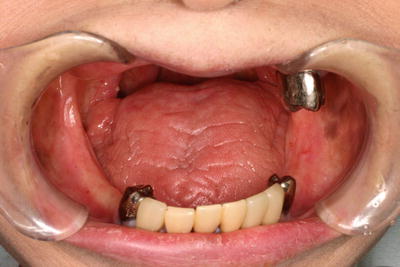
Fig. 15.3
Radiation-induced salivary gland dysfunction and taste disturbances. A patient with nasopharyngeal cancer who received external beam radiotherapy (70 Gy) with concurrent chemotherapy. Intraoral photograph indicates a dry mouth and a smooth tongue resulting from taste bud atrophy
Radiation-induced salivary gland dysfunction and taste disturbance are highly frequent acute or late adverse events that afflict most radiation patients similarly to oral mucositis. Salivary gland dysfunction is caused by ischemia following vascular occlusion and atrophy of the acinous cells [4, 5]. The degree of dysfunction depends on the radiation dose and the irradiated volume of the parotid gland; the dysfunction is nonreversible when the irradiated volume exceeds 75 % and the radiation dose is between 30 Gy and 45 Gy. Therefore, most external irradiation patients with oral cancer cannot recover after treatment [6]. Salivary dysfunction leads to oral adverse events, such as dental caries, periodontitis, and oral infections, which decrease the buffering capacity and antibacterial effect of saliva. Clinically, most patients complain about the viscosity of their saliva from day 3 and dry mouth from day 14 of radiotherapy; treatment with pilocarpine hydrochloride, a salivation accelerant, is prescribed. If the effects of pilocarpine hydrochloride are insufficient, a moisturizer is co-prescribed. Finally, if symptoms do not improve, herbal medicines such as byakkokaninjinto or bakumondoto may be prescribed. In recent years, mitigation of radiation-induced salivary gland dysfunction by a dose reduction to the parotid gland using intensity-modulated radiation therapy (IMRT) has been studied [7].
Radiation-induced taste disturbance is caused by atrophy of the taste buds by radiation [8] and is also affected by tongue coating and hyposalivation. Clinically, most patients complain of hypogeusia from day 7 of radiotherapy and some patients progress to ageusia from approximately day 14; near-normal taste levels are recovered 90–180 days after the end of irradiation [9] and only a few patients continue to have dysgeusia for over a year. Taste disturbance during radiotherapy does not only impair the patients’ quality of life but also their nutritional status and motivation for cancer treatment; appropriate supportive care, such as oral care instructions including tongue brushing and moisture retention of the oral cavity, is therefore required. Dietary modifications following the counseling of a nutritionist are also important. Finally, zinc sulfate, which aids taste bud cell regeneration, has been reported to accelerate the recovery from taste disturbance [10].
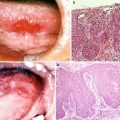
15.1.1.3 Radiation Caries and Periodontal Disease (Fig. 15.4)
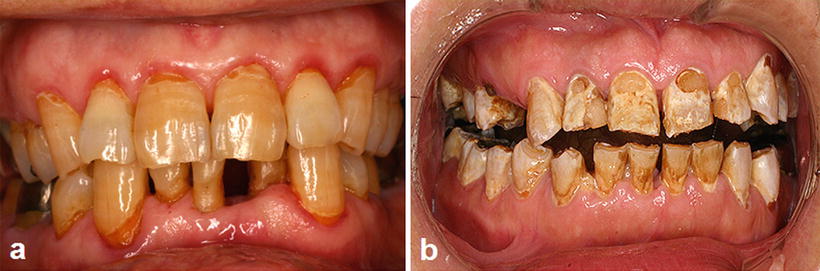
Fig. 15.4
Radiation caries and periodontitis. (a) A patient with tongue cancer who received postoperative external beam radiotherapy (60 Gy). Intraoral photograph 2 years after radiotherapy indicates multiple root surface caries, gingival swelling, and gingival redness. (b) Advanced case of radiation caries: a patient with floor of mouth cancer who received postoperative external beam radiotherapy (60 Gy). Intraoral photograph 6 months after radiotherapy indicates advanced multiple root surface caries
Radiation caries and periodontal disease are highly frequent late adverse events and are a major risk factor for osteoradionecrosis. Therefore, periodic oral and dental management after radiotherapy for their prevention is essential. Radiation causes the following changes in tooth and periodontal tissues: (i) a decrease in the microhardness of the dentin; (ii) gap formation at the enamel–dentin junction; (iii) derangement of periodontal ligament fiber; and (iv) cytopenia, hypovascularization, and fibrosis of periodontal tissues [11–13]. However, the main factor influencing radiation caries and periodontitis is thought to be radiation-induced salivary dysfunction given that the above radiogenic damages are mild [14].
The preferred sites for radiation caries are the occlusal surface and cervical region of the tooth, indicating that they occur on the exposed dentin. Cervical caries, in particular, must be carefully monitored since an attachment loss of approximately 1–2 mm develops after radiotherapy [15]. Radiation caries progress rapidly; DMFT has been reported to increase to 7.7 within 4 years after radiotherapy [16]. Following strong hypersensitivity symptoms on a tooth, the radiation caries turns into pulpitis. In addition, oral radiotherapy patients often present with trismus.
The use of topical fluoride applications is recommended to prevent radiation caries following head and neck radiotherapy. However, Spak et al. described that 80 % of patients with an unstimulated salivary flow rate of <0.1 mL/min may develop at least 1 carious lesion per year, despite daily applications of a fluoride gel [17]. Topical fluoride applications may not be able to completely control dental caries in irradiated patients with severe hyposalivation for the following reasons: (i) extended meal times due to dysphagia caused by hyposalivation, causing demineralization of the root surface to progress more during meals, and (ii) the calcium and phosphate required for remineralization are insufficiently supplied due to the considerable decrease in saliva. Remineralizing agents have been added to topical fluoride applications with an aim to improve caries control in hyposalivation patients [18, 19].
Radiation periodontal disease progresses as rapidly as radiation caries and causes tooth loss by resorption of the alveolar bone; poor oral hygiene caused by hyposalivation and periodontal tissue atrophy caused by radiation are its main causes. Radiation periodontal disease can be stabilized through good oral hygiene. However, inflammatory symptoms such as the swelling and redness of the gingiva may often be unclear due to hypovascularization of periodontal tissue caused by radiation. Therefore, periodical evaluation of periodontal tissue through dental and/or panoramic radiography is necessary.
15.1.1.4 Trismus
Trismus, or limited jaw mobility, has been reported in 6–86 % patients who received radiation to the temporomandibular joint and/or the masseter/pterygoid muscles. The different incidence rates are attributed to a difference in the observation period and evaluation methods. The prevalence of trismus after conventional radiation, IMRT, and chemoradiotherapy has been reported to be approximately 25 %, 5 %, and 30 %, respectively. In addition, the prevalence increases following a higher radiation dose (over 60 Gy). The main cause of trismus is fibrosis of the soft tissue included within the radiation field. The early treatment of trismus aims to minimize the limitation of jaw mobility after radiotherapy; treatment should begin upon the first presentation of clinical signs of impaired jaw mobility. Treatment consists of a forced jaw opening exercise using various appliances. The US National Cancer Institute recommends opening of the jaw as wide as possible without causing pain 20 times, 3 times a day.
15.1.1.5 Osteoradionecrosis of the Jaws (Figs. 15.5 and 15.6)
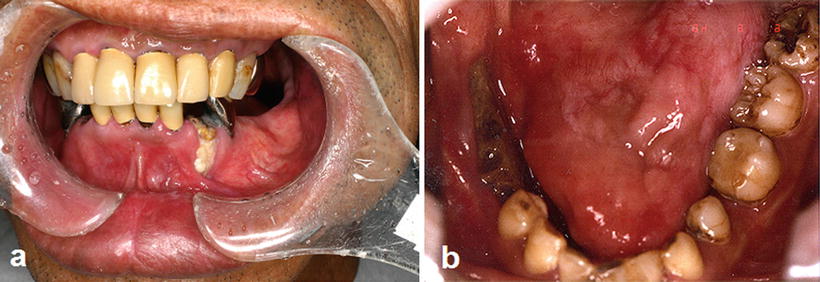
Fig. 15.5
Osteoradionecrosis of the jaw. (a) A patient with tongue cancer who received postoperative external beam radiotherapy (50 Gy). Intraoral photograph 5 years after radiotherapy indicates a severe gingival recession with bone exposure in the first premolar region. (b) A patient with tongue cancer who received interstitial radiotherapy (Cs-137, 75 Gy). Intraoral photograph 3 years after radiotherapy indicates bone exposure after extraction of the second premolar and first molar
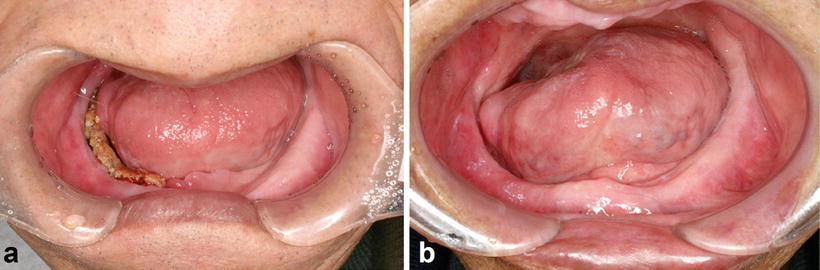
Fig. 15.6
Osteoradionecrosis of the jaw. A patient with tongue cancer who received interstitial radiotherapy (Cs-137, 75 Gy). (a) Intraoral photograph 5 years after radiotherapy indicates bone exposure induced because of the incompatibility of a complete denture. (b) Osteoradionecrosis healed by denture adjustment and conservative treatment without surgery 7 years after the onset of bone exposure
Osteoradionecrosis of the jaws is a rare late adverse event. However, patients suffering from osteoradionecrosis of the jaws experience a substantial deterioration in their quality of life due to serious clinical symptoms such as chronic spontaneous pain, dysphagia, and facial deformation.
The risk factors for osteoradionecrosis of the jaws are the location of the primary tumor relative to the jaws, the radiation dose received by the jaws, and oral conditions such as dental caries with periapical lesion, periodontal disease, and poor oral hygiene [20–23]; predominant risk factors are shown in Table 15.3. In addition, osteoradionecrosis of the jaws is classified into spontaneous and traumatic osteoradionecrosis. Endarteritis, hyperemia, hyalinization, cellular loss, hypovascularization, thrombosis, and fibrosis are the most common histological findings in spontaneous osteoradionecrosis [24]; the pathogenesis is described as hypovascular, hypocellular, and hypoxic tissue formation [25]. These changes are the direct, dose-dependent, and irreversible effects of radiation. Traumatic osteoradionecrosis of the jaws occurs for a long period after radiotherapy and is caused by dental caries with periapical lesion, periodontal disease (Fig. 15.5a), teeth extraction (Fig. 15.5b), poor oral hygiene, and inadequate denture irritation (Fig. 15.6); tooth extraction is the greatest risk factor. The most common reasons for tooth extraction are untreatable dental caries and periodontitis. Therefore, periodical oral management is essential to decrease the risk of dental caries and periodontitis. In fact, the incidence rate of osteoradionecrosis of the jaws is decreased to less than 10 % by systematic oral management before and after radiotherapy [26].
Table 15.3
Risk factors of osteoradionecrosis of jaws
Patient factors | Treatment factors |
|---|---|
Tooth extraction | Total radiation dose |
Teeth with periapical lesions | Radiation dose rate |
Inadequate denture | Radiation fraction size |
Oral hygiene | Radiation field size |
Tobacco | Radiation site |
Alcohol | Concurrent chemotherapy |
Poor nutritional status | Mandibular resection |
Tumor location | Neck dissection |
There is no established cure for osteoradionecrosis of the jaws, although conservative treatments such as local irrigation and antimicrobial administration are considered to be the safest. Segmental or marginal mandibulectomy combined with hyperbaric oxygen therapy can be performed if conservative treatment is difficult to perform. In this case, the patient’s quality of life is seriously impaired by dysfunction and deformity of the jaws.
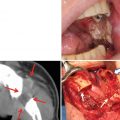
15.1.1.6 Hypoplasia of the Jaws and Dental Development Abnormalities (Fig. 15.7)
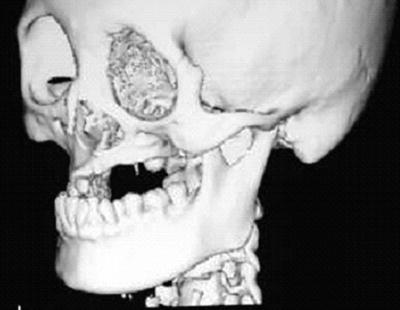
Fig. 15.7
A patient with osteosarcoma of the left maxilla who received external beam radiotherapy during childhood (details of radiotherapy are unclear). A three-dimensional reconstruction CT image indicates maxillary growth retardation and dental agenesis of the left side
Hypoplasia of the jaws and dental development abnormalities caused by radiation are limited to the radiation area. When patients receiving radiotherapy are younger, these abnormalities become more severe; the effects of radiation are stronger in patients aged 12 years or younger due to jaw growth and the formation of permanent teeth. Representative dental abnormalities caused by radiation are dental agenesis, microdontia, enamel hypoplasia, and shortened or tapering root. These adverse events do not only cause oral dysfunction and facial dysmorphic disorders but also mental distress in patients. Therefore, the long-term follow-up by a pediatrician, a psychiatrist, a plastic surgeon, and a dentist is required.
15.1.2 Planning and Management for Head and Neck Radiotherapy Patients
Oral and dental management for head and neck radiotherapy patients aims to prevent and alleviate acute and late oral adverse events in order to improve the patient’s overall survival rate and quality of life. Given that the treatments available for teeth included in the radiation field are limited, dentists and dental hygienists have to confirm or speculate the radiation field before dental treatment or management. Dental management for radiotherapy patients can be performed in three stages, namely before, during, and after radiotherapy. These stages aim to prepare the base for alleviation and prevention of acute and late oral adverse effects and improve the patient’s quality of life. If the pre-radiotherapy stage is not conducted appropriately, subsequent stages cannot proceed smoothly. For reference, Table 15.4 shows the summary of our oral management protocol for radiotherapy patients. Considering the increasing number of cancer survivors, it is expected that dental management after radiotherapy will soon be provided in general dental clinics.
Table 15.4
Summary of our oral management protocol for patients receiving head and neck radiotherapy
Stage I | Stage II | Stage III |
|---|---|---|
Before radiotherapy | During radiotherapy | After radiotherapy |
Patient education Oral care instruction Removal of oral risk factors for radiation mucositis (refer Table 14.2) Extraction of teeth with poor prognosis (particularly, teeth within the radiation field) Untreatable teeth Acute symptomatic teeth with apical lesions Teeth requiring surgical endodontics Advanced periodontal disease (>6 mm pocket depth and >60 % alveolar bone loss) Partially erupted third molar Temporary dental treatment Spacer production | Self oral care Oral cleaning (3 times/day) Mouth rinsing and mouth moisture retention (8 times/day) Denture cleaning (3 times/day) Professional oral care (once/week) Oral examination Oral cleaning Symptomatic treatment | Preventive dental maintenance 9,000 ppm fluoride application (every 3 months) Periodontal examination and scaling (every 6 months) Radiography examination (every 12 months) Resumption of dental treatment |
15.1.2.1 Oral and Dental Management Before Radiotherapy
The purpose of oral and dental management before radiotherapy is to lay the base for alleviation and prevention of acute and late oral adverse events. It consists of patient education and oral conditioning for head and neck radiotherapy. Ideally, oral and dental management including dental treatment should start within 2–3 weeks before radiotherapy.
Patient Education
Patient education aims to elucidate the oral adverse events caused by radiotherapy (appearance day, degree, duration, and negative effects on cancer treatment and patient’s quality of life) and to instruct on their prevention and alleviation (oral self-care and interventional schedule of professional oral care). Since patient education is the most important factor in the prevention and alleviation of oral adverse events, the healthcare provider must take care to illustrate these comprehensibly. In addition, building a good rapport between medical care providers and patients is also an essential condition for a successful outcome.
Oral and Dental Conditioning
Oral and dental conditioning aims to remove the oral risk factor of radiation mucositis and osteoradionecrosis, as well as the oral sources of infection. Because the extraction of a tooth included within the radiation field is contraindicated after radiotherapy in principle, an untreatable tooth within the radiation field must be extracted before radiotherapy (Table. 15.4). In addition, when radiotherapy is started before the tooth extraction wound epithelializes, the immature granulation tissue will often slough off, causing osteoradionecrosis. Therefore, it is recommended that tooth extraction is performed at least 14 days before the start of radiotherapy and that the wound is fully closed.
Intraoral orthodontic appliances and metal dental restorations cause dose escalation at the surface of the oral mucosa due to backscattered radiation and are thus specific risk factors for radiation mucositis (Fig. 15.2). Therefore, an orthodontic appliance must be removed before the commencement of radiotherapy. Nevertheless, the removal of dental metal restorations such as full metal crowns incurs problems given that the time from their removal to the provisional restoration is limited as well as a financial burden due to the need of remanufacture after removal of the restoration is high. A spacer can be used to resolve these problems (Fig. 15.8), as it can eliminate the need for the removal of the metal dental restoration. Since the influence of the backscatter radiation is within the range of 3–5 mm from the surface of the metal dental restoration, the spacer thickness should be more than 3 mm [27].
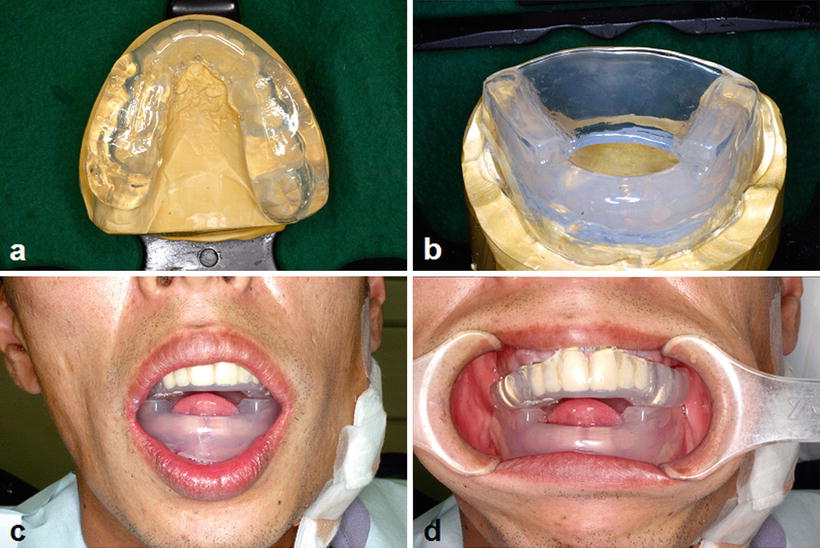

Fig. 15.8
Spacer provided to protect oral mucosa from the backscatter radiation. (a) Spacer for the upper jaw. (b) Spacer for the lower jaw with tongue-depressing plate. (c, d) Spacer in appropriate position. Note the anterior positioning of the tongue by the patient
15.1.2.2 Oral and Dental Management During Radiotherapy
The main purpose of oral and dental management during radiotherapy is to prevent and alleviate radiation mucositis and radiation xerostomia, although there is no established treatment method for these. Therefore, supportive treatment such as preventive oral care and symptomatic treatment are mainly performed. Preventive oral care consists of self-care and professional care by a well-trained dental health provider. Self-care consists of toothbrushing, tongue and oral mucosa cleaning, mouth rinsing, and mouth moisture retention. Professional care consists of the examination of the oral status, oral care instructions, and professional oral cleaning. Since clinical changes in the oral conditions caused by radiation occur on a weekly basis, professional oral cleaning should be performed weekly from the start of radiotherapy to the disappearance of radiation mucositis. Symptomatic treatment consists of moisturizing instructions and pain management.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree