Radiologic Evaluation of Allergic and Related Diseases of the Lower Airway
Thomas Grant
The radiographic appearance of thoracic manifestations of systemic immunologic and allergic disorders comprises a variety of abnormalities that are influenced by the pathophysiologic characteristics of the underlying disease. These disorders include collagen vascular diseases and the systemic vasculitides. The clinician must also be familiar with the diseases in this category, a diverse group including Churg-Strauss syndrome (CSS), Wegener granulomatosis, asthma, allergic bronchopulmonary aspergillosis (ABPA), bronchocentric granulomatosis, hypersensitivity pneumonitis, eosinophilic lung disease, and drug induced lung injury.
Immunologic and allergic diseases of the lungs can manifest radiographically as diffuse or focal pulmonary parenchymal and airway abnormalities (1,2). In earlier stages of the disease, radiographs may be normal. Although chest radiographs are usually abnormal in advanced disease, characterization is frequently impossible. Thin section, or high resolution computed tomography (HRCT) has become the most important imaging technique to confirm the diagnosis.
Computed Tomography
Recent developments using multi-detector CT (MDCT) change the way in which radiologists perform and interpret studies of the thorax (3,4). Current generation CT scanners with 16 or more detector rows can acquire isotropic volumetric data in a single breath hold. For example, MDCT scanners can cover the entire chest with a <1 mm collimation (section of thickness) in less than 20 seconds. MDCT not only generates traditional axial images but can acquire volumetric data sets to view the computer-generated information in multiple nonaxial planes if desired (4). The volumetric acquired data make CT ideal for detecting, characterizing, and distinguishing among diseases of the lungs, mediastinum, and pleura.
HRCT involves obtaining narrow (<2 mm) collimation, a small field of view, and a high spatial frequency reconstruction algorithm to obtain detailed images of the lungs. By using a very thin section, structural superimposition within the section of thickness is reduced, permitting optimal evaluation of lung detail. At our institution, the HRCT is typically performed with the patient in the supine position. Images are obtained in full inspiration from the apex to the diaphragm using 1.0 mm collimation. This is followed by a fewer number of inspiratory images obtained in a prone position. Expiratory images are obtained to look for air trapping. HRCT can detect lung disease at an early and potentially treatable stage. HRCT is also valuable in differentiating acute from chronic disease and optimizing the site for bronchoscopic or open lung biopsy (3–5). The HRCT findings of certain immunologic lung diseases are often so characteristic that a lung biopsy is not required (6,7).
The use of intravenous contrast is frequently unnecessary for chest CT examinations, particularly for evaluating pulmonary nodules or interstitial lung disease. However, intravenous contrast can help to distinguish lymph nodes from pulmonary vessels, characterize pleural disease, demonstrate vascular components of an arterial venous malformation, and detect pulmonary emboli.
Intravenous contrast should be avoided in patients with a creatinine level above 1.5 mg/dL, in patients with multiple myeloma, and in patients with suspected pheochromocytoma. Low osmotic contrast is now preferred because it has fewer side effects. Corticosteroid pretreatment supplemented with antihistamine, diminishes the risk of adverse reactions in patients with a previous anaphylactoid reactions to contrast material.
Computed Tomography Anatomy
The lung is composed of lobes, segments, subsegments, secondary lobules, and acini (8,9). Each level contains an airway, a pulmonary artery, and a supporting structure, the peribronchovascular interstitium. The airway is a branching series of 20 generations that leads to the alveoli.
The secondary pulmonary lobule is the smallest unit of lung structure marginated by connective tissue septa (10). These lobules measure 1 cm to 2.5 cm and are supplied by a small bronchial and pulmonary artery. The secondary lobule can be identified in both normal and abnormal lungs. There are distinct patterns of abnormality on HRCT that help to define acute and chronic infiltrative lung diseases (Tables 11.1 and 11. 2).
Table 11.1 Common Computed Tomographic (CT) Features of Immunologic Lung Diseases | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 11.2 Computed Tomographic (CT) Appearance Of Immunologic/Eosinophilic Lung Disease | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
Reticular opacities result from thickening of the pulmonary interstitium by fluid, fibrosis, or other materials. Pulmonary fibrosis on HRCT is characterized by honeycombing. In usual interstitial pneumonia honeycombing is most often observed peripherally at the lung bases (5,7). In chronic hypersensitivity pneumonitis the fibrosis is usually most severe in the mid or upper lung zones (11,12). Cysts or rounded air-containing nodules are present in a number of acute and chronic infiltrative diseases. Nodules in a centrilobular distribution that involve the secondary pulmonary lobule can be seen in subacute hypersensitivity pneumonitis, pulmonary hemorrhage, cryptogenic organizing pneumonia, nonspecific interstitial pneumonia, and respiratory bronchiolitis-associated interstitial lung disease (13).
Ground-glass attenuation is characterized by the presence of hazy increased attenuation of lung without obscuration of the underlying bronchial or vascular anatomy. If the vessels are obscured, the term consolidation is used. Ground-glass attenuation can result from interstitial thickening, air space filling, or both. Although ground-glass attenuation is nonspecific, it usually indicates the presence of an active, potentially treatable disease.
Radiologic Findings in Immunologic Lung Disease
Churg-Strauss Syndrome
CSS is a rare allergic necrotizing vasculitis of unknown etiology (15–17). The syndrome is most commonly seen in patients 30 years to 50 years of age and has no gender predilection. Patients are typically asthmatic and present with eosinophilia, fever, and multisystem vasculitis. Findings of chest radiography are often abnormal and most often consist of patchy nonsegmental areas of consolidation with no zonal predominance. The areas of consolidation may have peripheral distribution and are often transient. A pleural effusion is present in approximately 30% of patients, usually due
to cardiac involvement or eosinophilic pleuritis (15). Up to 25% of patients with CSS have few or no imaging abnormalities.
to cardiac involvement or eosinophilic pleuritis (15). Up to 25% of patients with CSS have few or no imaging abnormalities.
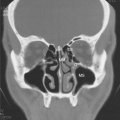
Recent reports on the HRCT findings of CSS have shown that they are nonspecific. Findings include ground-glass opacities, consolidation, small centrilobular nodules, interlobular septal thickening, and bronchial wall thickening (Fig. 11.1




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree