Major Concepts
Incidence
Tuberculosis (TB) is an ancient enemy, plaguing humans for over 7,000 years and affecting every region of the world. Some populations are afflicted more than others, with socioeconomic factors playing a large role in disease prevalence in that crowded living conditions and poor sanitation contribute to transmission of the causative bacterium. Latin America, Africa, and Asia have high incidence of infection. In the United States, residents of homeless shelters, migrant farmworker camps, prisons, some nursing homes, mental health facilities, and large urban centers have higher risk of infection. Infection is also more common among Hispanics and persons of African descent than among Caucasians. Disease incidence dramatically decreased in the 1940s due to increased sanitation, improved general health, higher socioeconomic status, and the discovery of effective chemotherapeutic drugs. The victory was temporary, however, and infection numbers rose again in the mid-1980s. Weakened immune status due to the AIDS pandemic, combined with inadequate health care in many regions of the world and the spread of drug-resistant strains of bacteria, contributed to TB’s reemergence. Currently, 2 to 3 million deaths occur each year, making TB the single leading infectious cause of death in persons older than 5 years. One-third of humanity is latently infected with the TB bacilli—a huge reservoir of potential bacterial transmission.
Symptoms
TB is typically acquired by inhaling bacilli found in small aerosolized respiratory droplets produced when persons with active disease cough, sneeze, talk, laugh, or sing. Oral transmission previously played a large role in the spread of disease, but has greatly decreased due to declining numbers of infected cattle and routine pasteurization of milk. Following airborne infection, bacteria enter the lungs, are ingested by alveolar macrophages, escape destruction in the phagolysosome, and multiply in the cell’s cytoplasm or extracellularly. The ensuing inflammatory reaction causes immune cells to wall off the area, forming tubercles. Cells and bacilli then disintegrate, forming a homogeneous, coagulated mass that may persist for prolonged periods of time. The area is often encapsulated by collagenous material, slowing bacterial growth and containing the infection, which then passes into a latent form. In 5% to 15% of infected individuals, active disease develops at some later time due to reactivation of formerly dormant tubercles. The lesion’s center liquefies and oxygen-rich air may flow into the cavity, rapidly accelerating bacterial growth. Bacilli disseminate throughout the lungs and may spread to other organs, including the lymph nodes, bones and joints, kidneys, or brain. Symptoms of active TB are chronic cough, dull and aching chest pain, coughing up of blood or sputum, shortness of breath, malaise, weakness and fatigue, weight loss, loss of appetite, chills, fever, and severe night sweats. The mortality rate of untreated active disease is 50%.
Infection
TB is caused by several species of mycobacteria, usually Mycobacterium tuberculosis, but M. bovis, the M. avium complex (MAC), M. africanum, or M. microti may also cause the disease. All these bacteria are nonmotile, noncapsulated, non-spore-forming, acid-fast bacilli that prefer environments similar to alveolar air. Mycobacteria are slow-growing organisms with a unique colony appearance and unusual growth requirements in vitro.
Treatment and Resistance
Treatment of TB involves long-term administration of combination therapy with drugs such as streptomycin, isoniazid, rifampin, pyrazinamide, para-aminosalicylic acid, ethambutol, viomycin, kanamucin, or capreomucin. Drug resistance rapidly arose, first to streptomycin and then to isoniazid and rifampin. Mycobacterial strains resistant to both rifampin and isoniazid and sometimes other drugs are designated as multidrug-resistant TB (MDR-TB). Rates of MDR-TB increased sharply in the early 1990s due to the dismantling of public health infrastructure, increased numbers of persons in homeless shelters or prisons, the AIDS epidemic, inadequate infection control procedures in hospitals, and poor practices in prescribing and compliance with anti-TB medications. MDR-TB is now a global concern. Extensively drug-resistant TB (XDR-TB) is also increasing in prevalence and is resistant to at least rifampin, isoniazid, one of the second-line injectable drugs, and a fluoroquinolone. XDR-TB has a much higher mortality rate than TB or MDR-TB.
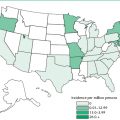
Tuberculosis (TB; also formerly known as consumption) has plagued humankind since antiquity. Once the leading cause of death in the United States, this dreaded disease was dramatically curbed by the discovery of several new drugs in the 1940s. The respite was short-lived, however, and in 1984, TB incidence began to increase again; greater than 25,000 cases were reported in the United States in 1993. The AIDS pandemic and the consequent weakening of large number of persons’ immunity have fueled this reemergence, and many TB cases (57%) are found in California, Florida, Illinois, New York, and Texas, where HIV infection rates are high. Throughout the world, 2 to 3 million TB-related deaths occur annually and 8 to 12 million new cases are identified each year. It is currently the single leading cause of death due to infectious disease in individuals over the age of 5 years. Approximately one-third of the world’s population is thought to harbor latent TB infection.
TB strikes certain populations more commonly than others. The infection rate in men is twice that in women. Crowded living conditions and poor sanitation contribute to increased incidence of this disease. It is especially common among persons living in homeless shelters, migrant farmworker camps, prisons, and some nursing homes and mental health facilities. TB is particularly problematic throughout Latin America, Africa, and Asia. In the United States, residents of large urban centers are at high risk. In these areas, the infection rate among Caucasians is lower than that among persons of Hispanic or African descent. Socioeconomic factors play an important role, with the least affluent members of society being the most vulnerable.
Throughout recorded history, tuberculosis has left its deadly mark. Human bones dating to 5000 B.C. have shown infection. Egyptian mummies also show disease manifestations. Hippocrates described a disease called phthisis (“wasting away”), characterized by lung nodules that resembled the tubercles of TB, in 1000 B.C. The major causative bacterium in humans, Mycobacterium tuberculosis, was discovered by Robert Koch in 1882, and two additional species, M. bovis and M. avium, were recognized by 1900. These also cause disease in cattle and birds. Several other related atypical types of mycobacteria also causing lung disease were discovered by the 1950s.
TB has left a trail throughout the history of the Western world; it was a common cause of death from the Middle Ages until the early 1800s. During the seventeenth and eighteenth centuries, it is believed to have caused 20% to 30% of the deaths in London. Brought to the Americas by European colonists, TB soon wreaked havoc in the New World. In several large U.S. metropolitan areas, TB infection was responsible for 300 to 400 deaths per 100,000 population between 1812 and 1880; the toll decreased to 245 per 100,000 in 1900, 119 in 1920, 69 in 1932, and just 5 in 1970. Much of the decrease in numbers of infected individuals and deaths, especially in developed areas, resulted from increased sanitation, general health, and socioeconomic status followed by the discovery of effective chemotherapeutic drugs. Incidence increased again in the 1990s due in part to the AIDS pandemic, lack of adequate health care in portions of the world, and increased prevalence of drug-resistant strains.
At various times in the nineteenth and twentieth centuries, sanatoriums became popular among people who were able to afford them. Patients in these institutions lived for extended periods of time in isolation, receiving bed rest, sunshine, and nutritious foods. Exposure to fresh, often cool, air was stressed, and sanatoriums were therefore often located in alpine settings, where patients sat outside covered with blankets. Mammoth Cave, with its chill environment, once housed a sanatorium. In 1892, Hermann Briggs, the New York City health commissioner, implemented a program to decrease TB in that city by reporting cases, stressing proper disposable of lung discharges, and education.
TB influenced and affected many aspects of human history. In the arts, the heroines of La Bohème and Moulin Rouge suffered from this disease. Dostoevski’s Crime and Punishment and Upton Sinclair’s Jungle both featured TB. A number of prominent people succumbed to this infection, including Frédéric Chopin, Henry Thoreau, John Keats, Emily Brontë, Elizabeth Barrett Browning, Robert Burns, Alexander Pope, Cardinal Richelieu, John Calvin, Andrew Jackson, Eleanor Roosevelt, Alexander Graham Bell, and Louis Braille.
Although TB is usually associated with infection of the lungs, it may attack any part of the body. Because this disease generally relies on airborne transmission, bacteria settle initially in the lungs and may later spread via the circulatory system to other organs, such as the kidneys, lymph nodes, joints, spine, or brain. Transmission usually occurs by inhalation of aerosolized droplets containing the causative species of mycobacteria. Such droplets are produced by individuals with active lesions when they cough, sneeze, talk, laugh, or sing. Those droplets containing viable bacilli, which are only a few micrometers in diameter, may remain suspended in the air for up to 30 minutes. Only a few bacteria are needed to initiate infection via this route. Larger particles tend to deposit in the upper respiratory tract and are often expelled. Other means of transmission are currently much less common, and the bacteria are readily killed by drying or exposure to sunlight. These characteristics inhibit the usefulness of mycobacteria as bioterrorist agents. Crowded living conditions and poor hygienic practices, including failure to cover one’s mouth when coughing or sneezing, facilitate transmission. Transmission via the oral route through the ingestion of cow’s milk was far more common in the past until tuberculosis in cattle was brought under control and milk was routinely pasteurized. Infection via this route requires ingestion of a large number of bacteria.
Once in the body, bacilli travel to the alveoli of the lungs, where some are phagocytized by alveolar macrophages and multiply in their cytoplasm. Other bacteria multiply outside the cells. Infection induces an inflammatory reaction, and the alveoli fill up with fibrin, additional macrophages, and neutrophils (other phagocytic immune system cells). Bacteria may escape from the local focus and travel via the pulmonary lymph nodes to other areas of the lungs. The neutrophils are later replaced by more macrophages, and lymphocytes are recruited into the area. Small inflammatory lesions then evolve into productive walled-off areas named tubercles with a peripheral region of lymphocytes and a central area of Langerhans cells, epithelioid cells, and multinucleated giant cells. The latter two cell types are derived from activated macrophages that have altered their shape to become epithelioid cells, some of which then fuse to form giant cells. The few bacilli present dwell in the epithelioid cells. Formation of multinucleated giant cells also occurs during HIV infection. Later, as a delayed-type hypersensitivity reaction develops, caseation may occur, characterized by the disintegration of cells and bacilli and the formation of a homogeneous, coagulated mass with a cheeselike appearance that may persist for years. Often the area undergoes inspissation, becoming encapsulated by collagenous material, and bacterial growth rate slows as the infection is contained by the host’s immune response. During subsequent years, calcium salts may deposit in these lesions, forming Ghon complexes that may be detected by chest X-rays.
In some individuals, infection may later reactivate in formerly dormant caseous lesions and progress to the liquefaction stage of chronic cavitary pulmonary TB, in which the centers of the tubercles becomes liquid, providing a rich growth medium for the bacteria and accelerating their growth. Often the bronchus erodes as the surrounding lung tissue becomes involved. The liquid contents of the tubercle then drain out of the lesions, and the tubercle may subsequently undergo cavitation, in which the center is transformed into an air-filled abscess, the tuberculous cavity, and exposure to oxygen-rich air leads to rapid bacterial growth.
Under normal conditions, primary lesions and noncaseous tubercles heal spontaneously. After caseation, however, lesions seldom resorb. If the primary lesions are not localized, the disease may disseminate, spreading through the lungs. A heavy load of bacilli in the lungs may enter the lymphatic and circulatory systems, spreading throughout the body and leading to the development of miliary TB lesions in other organs, including lymph nodes, bones and joints, and the kidneys. When such lesions occur in the brain or meninges, the extremely serious tubercular meningitis may develop.
Most infections with TB assume a latent form, and only 5% to 15% progress to active infection. In its latent form, TB causes no symptoms and the individual does not feel ill. Although these latently infected people do react positively for TB by the commonly used skin test, they are not able to spread the infection. Such individuals may later develop active TB without proper treatment and serve as potential disease reservoirs.
Some individuals rapidly develop an active form of the disease after their initial infection. Such progressive primary tuberculosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree