Introduction
Respiratory insufficiency in patients receiving systemic chemotherapy often is a diagnostic dilemma for clinicians. Patients receiving chemotherapy are frequently immunosuppressed and may have concurrent bone marrow suppression, or pulmonary involvement from underlying malignancy, making the differential diagnosis of respiratory failure quite broad. It is imperative that common etiologies such as respiratory infections, congestive heart failure, venous thromboembolism, alveolar hemorrhage, radiation-induced lung injury, and toxicities from chemotherapeutic drugs be investigated in cancer patients presenting with respiratory failure. As drug toxicity is a diagnosis of exclusion and cannot be defined by uniform diagnostic criteria, an extensive workup, sometimes including a lung biopsy, is required to make a diagnosis. Although the exact incidence of lung injury from chemotherapeutic agents is unknown, some authors suggest that it may be as high as 10% to 20%. , It is important to recognize the clinicopathologic features that are characteristically associated with different antineoplastic agents and have a high degree of suspicion to allow for early intervention and avoidance of any further exposure to the offending agent.
Pulmonary toxicities of chemotherapeutic agents are mediated through many different mechanisms and can have varied clinical manifestations. In addition, novel targeted agents and immunotherapeutic agents are associated with very distinct pulmonary toxicities in comparison with traditional chemotherapeutic drugs. In this chapter, we will be focusing on pulmonary toxicities of cytotoxic chemotherapy.
- ■
Pathophysiology: Several mechanisms have been suggested for lung injury from cytotoxic agents; however, the majority of these are poorly understood. Cytotoxic chemotherapy can result in direct injury to alveolar pneumocytes resulting in a chemical pneumonitis-like pattern. Cytokines appear to play a prominent role in mediating pulmonary toxicities of these agents. Damage to endothelial cells in the lungs can lead to the release of cytokines, which in turn leads to activation and infiltration of inflammatory cells such as lymphocytes and, occasionally, eosinophils. Preclinical mouse models receiving gemcitabine and radiation (a known risk factor for gemcitabine-induced pneumonitis), have shown elevated levels of proinflammatory cytokines such as TNF-α and IL-1α. A similar increase in TNF-α and IL-1β has been noted in preclinical animal models as well as in humans who receive bleomycin. Fibroblast activation by both bleomycin itself and associated cytokine production can lead to collagen deposition. A systemic cytokine-mediated capillary leak syndrome resulting in noncardiogenic pulmonary edema has been described with gemcitabine and docetaxel. Free radical–mediated endothelial injury appears to play a prominent role in bleomycin-induced lung injury and has led to the investigation of the therapeutic utility of agents such as dexrazoxane and amifostine in mitigating the damage caused by bleomycin. In addition, other medications or radiation may have synergistic mechanisms which lead to augmented lung damage when used in combination. One such example is the increased incidence of pneumonitis in patients who are treated with gemcitabine and who have previously received radiation.
- ■
Risk factors: Patients who have underlying lung disease such as interstitial lung disease, chronic obstructive pulmonary disease (COPD), or other conditions, or those who are receiving a combination of cytotoxic agents or other drugs that can be associated with pulmonary toxicities, appear to be at an increased risk of developing pulmonary toxicities. In addition, prior or concurrent thoracic radiation puts patients at an increased risk for pneumonitis with certain agents such as gemcitabine. Exposure to high fractions of inspired oxygen (FiO 2 ) has also been well-known to amplify the risk of pneumonitis from bleomycin.
- ■
Clinical presentation: Although clinical trials often report pulmonary toxicities of chemotherapeutic agents, the exact clinicopathological presentations are often not described. Whereas some agents are associated with certain classic clinical findings such as bleomycin-induced pneumonitis, others can be associated with nonspecific signs and symptoms at presentation. Clinical manifestations are often nonspecific and include cough, dyspnea, hypoxia, and, occasionally, fever. Other specific signs and symptoms associated with the different clinical syndromes have been described below. Although most of these reactions occur within hours to weeks of initiating therapy, some drugs, such as bleomycin and nitrosureas, have been associated with delayed lung toxicity. ,
Subtypes of Chemotherapy-Associated Pulmonary Toxicity
- ■
Mast cell-mediated toxicity: Some agents, such as platinum drugs, taxanes, rituximab, cytarabine, and etoposide, have been associated with acute bronchoconstriction leading to dyspnea and hypoxia either during infusion or shortly thereafter. These infusion reactions are likely mediated by mast cell or basophil activation and can be associated with other systemic signs such as angioedema, hypotension, flushing, pruritis, and urticaria. These patients have prominent wheezing on examination and spirometry reveals severe reversible airflow obstruction.
- ■
Hypersensitivity pneumonitis: Towards the other end of the spectrum of allergic reactions are eosinophilic pneumonitis and hypersensitivity pneumonitis. Hypersensitivity pneumonitis is usually a cell-mediated process similar to delayed type IV hypersensitivity reactions. Patients with hypersensitivity pneumonitis develop dyspnea hours to days after receiving the cytotoxic drug. Thoracic imaging reveals new pulmonary infiltrates which may be associated with peripheral eosinophilia. Eosinophilic pneumonia often has diffuse alveolar or mixed alveolar-interstitial opacities on imaging and is characterized by greater than 20% eosinophils on analysis of bronchoalveolar lavage (BAL) samples. Peripheral eosinophilia is often present.
- ■
Interstitial pneumonitis: Interstitial pneumonitis often presents with diffuse or focal ground glass opacities ( Fig. 8.1 ) on imaging and septal thickening. Patients may have systemic symptoms such as fever. BAL findings in these patients can be nonspecific.
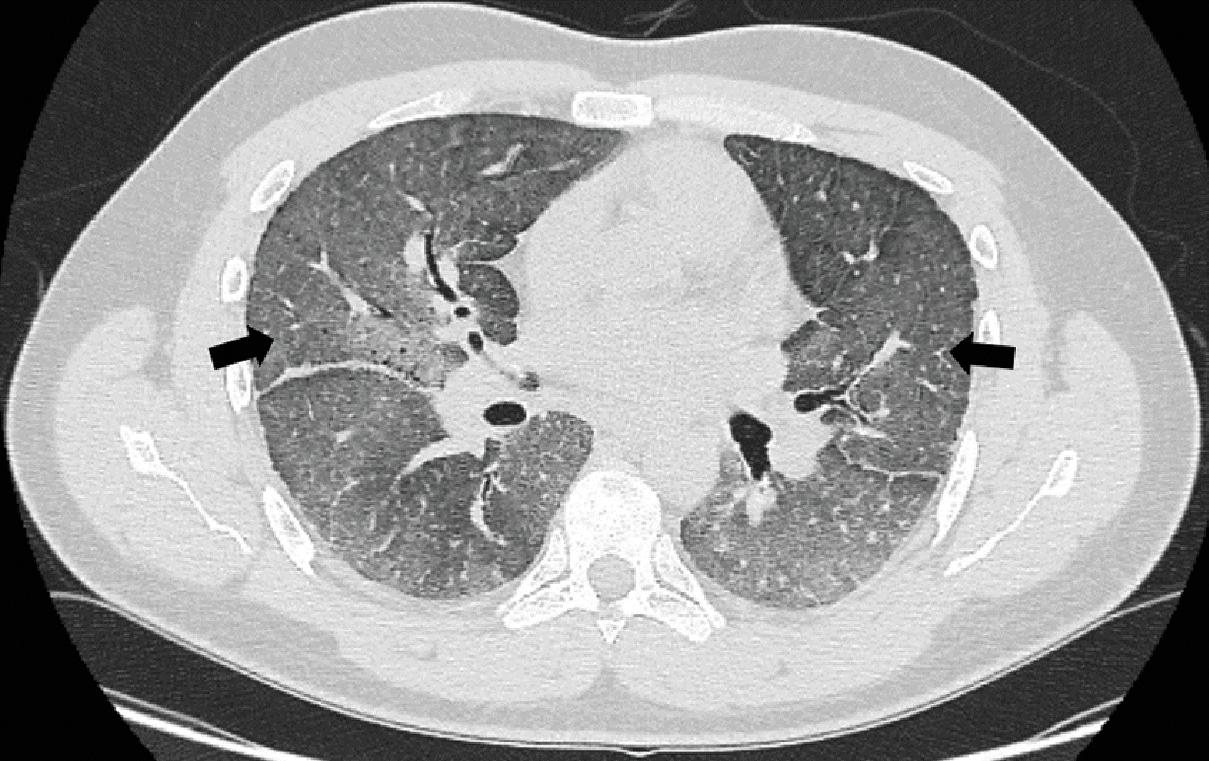
Fig. 8.1
Diffuse ground glass opacities noted on computerized tomogram of the chest (black arrows) in a patient receiving bleomycin.
- ■
Alveolar hemorrhage: Patients with alveolar hemorrhage present with dyspnea, occasionally associated with hemoptysis and diffuse pulmonary opacities on imaging. Diagnosis can be confirmed by performing a bronchoscopy and BAL, which will reveal a hemorrhagic sample.
- ■
Noncardiogenic pulmonary edema: Noncardiogenic causes of pulmonary edema such as capillary leak syndrome (associated with peripheral edema and, occasionally, intravascular volume depletion) should be considered in patients presenting with respiratory compromise who are receiving certain drugs such as cytarabine, gemcitabine, and docetaxel, among others.
- ■
Differentiation syndrome: Up to a quarter of patients with acute promyelocytic leukemia (APL) who are treated with either all-trans retinoic acid (ATRA) or arsenic trioxide (ATO) can develop a potentially fatal differentiation syndrome, due to the release of inflammatory cytokines as a result of differentiation of the promyelocytes to more mature neutrophils. These patients usually develop acute respiratory failure, fever, peripheral edema, pulmonary opacities, hypoxemia, hypotension, renal and hepatic dysfunction, rash, and serositis resulting in pleural and pericardial effusions.
- ■
Radiation recall pneumonitis: In patients who have received prior thoracic radiation, radiation recall pneumonitis (RRP) in the form of infiltrates in the region of previous radiation exposure has been noted. Drugs that have been associated with this phenomenon include doxorubicin, gemcitabine, paclitaxel, carmustine, etoposide, and trastuzumab.
- ■
Veno-occlusive disease: Pulmonary veno-occlusive disease (VOD) is another rare clinical presentation in which patients develop pulmonary hypertension, centrilobular ground glass opacities, septal lines, and lymphadenopathy on imaging.
- ■
Acute respiratory distress syndrome: Acute lung injury or acute respiratory distress syndrome (ARDS) has been observed with bleomycin, cytarabine, gemcitabine, mitomycin, and dactinomycin. Patients present with moderate to severe hypoxemia associated, on occasion, with systemic signs such as fever. BAL often shows a predominance of neutrophils.
Diagnostic Workup of Suspected Chemotherapy-Associated Pulmonary Toxicity
The diagnosis of cytotoxic chemotherapy–related pulmonary toxicity is one of exclusion. Careful attention must be paid to medical history, concomitant medications, prior or current thoracic radiation, and accompanying signs and symptoms, in order to rule out other etiologies such as infection, cardiogenic pulmonary edema, alveolar hemorrhage, pulmonary embolism, or a reaction to another medication. Often the diagnosis of drug-induced pneumonitiis is made when other diagnoes are excluded an there is an improvement in the clinical status of the patient after discontinuation of the drug with or without additional treatment such as corticosteroids.
- ■
Imaging: Chest imaging in the form of a chest X-ray or high-resolution computed tomography (CT) scans, although not specific, can be informative in recognizing the patterns of pulmonary toxicity, and to rule out other etiologies such as venous thromboembolism. A variety of abnormalities such as diffuse or focal ground glass opacities (see Fig. 8.1 ) or reticular markings, consolidations, centrilobular nodules, septal thickening, or pleural effusions associated with serositis may be seen. A classic radiographic pattern associated with methotrexate is the appearance of hilar lymphadenopathy.
- ■
Laboratory analysis: Laboratory values for the white blood cell count (neutrophilia, lymphocytosis, or eosinophilia) and inflammatory markers such as the erythrocyte sedimentation rate or C-reactive protein may be elevated in some patients. Other testing, which may be considered, based on clinical presentation include a B-type natriuretic peptide (BNP), coagulation studies, and echocardiogram, along with blood and sputum cultures. Although not used in routine clinical practice yet, elevated serum levels of Krebs von den Lunge-6 (KL-6), which is expressed by type II pneumocytes, may be seen in patients with drug induced pneumonitis.
- ■
Bronchoscopy: Bronchoscopy and BAL can play an important role in excluding other etiologies such as infection, involvement with malignancy, and hemorrhage. In some patients, a predominantly neutrophilic or eosinophilic BAL specimen may aide in the diagnosis of drug-induced pneumonitis. A lung biopsy can provide more information about the pathophysiology in a patient with respiratory failure; however, the histologic findings are often nonspecific and the risk versus benefit of the biopsy must be considered in each patient. Patterns observed on histopathology may point towards a diagnosis of drug-induced organizing pneumonia, nonspecific interstitial pneumonia, eosinophilic pneumonia, or pulmonary fibrosis.
- ■
Pulmonary function testing: Pulmonary function tests can be used to determine the degree of respiratory compromise; however, they are not useful in making a diagnosis.
Cytotoxic Chemotherapy Agents and Mechanisms of Action
ANTINEOPLASTIC ANTIBIOTICS
- ■
Bleomycin: Since it was first isolated in 1966, bleomycin has gained widespread application in the management of many malignancies including Hodgkin’s lymphoma and germ cell tumors. However, an estimated 10% of treated patients develop pulmonary toxicities, which often limits the clinical utility of the drug. Fatal pulmonary toxicity has been noted in up to 3% of patients. , Long-term toxicities have been reported in 8% of patients who received three courses of BEP (bleomycin, etoposide, cisplatin) and in 15% to 18% of patients treated for Hodgkin’s lymphoma. With early discontinuation of bleomycin determined by decline in diffusing capacity of the lungs for carbon monoxide (DLCO), more recent data from the Danish Testicular Cancer database have reported much lower rates of pulmonary toxicity.
- ■
Risk factors: Bleomycin lung toxicity appears to be more common with advanced age, , although some studies have not found an association. Cumulative drug dose above 400 units has been associated with higher rates of pulmonary toxicity and is generally avoided. Since bleomycin is mostly renally eliminated, patients with renal impairment are at an increased risk of drug accumulation and toxicity. Other factors that have been associated with higher pulmonary toxicity, albeit not consistently, include faster infusion rates, other concomitant chemotherapeutic agents such as cisplatin and gemcitabine, , thoracic radiation, , smoking, , and administration of granulocyte colony stimulating factors. The association between bleomycin lung toxicity and high FiO 2 has been shown in animal models; however, the data in humans is limited to retrospective case series and studies.
- ■
Mechanism: Bleomycin induces cytotoxicity by causing single and double stranded breaks in DNA by forming a complex with ferrous ions and molecular oxygen. After administration, it is rapidly inactivated in tissues by bleomycin hydrolase, especially in the liver and kidney. Cells in the lungs and skin are relatively deficient in this enzyme and are therefore more susceptible to bleomycin toxicity. Pulmonary toxicity by bleomycin is thought to be mediated by various pathways. In murine models, lower bleomycin hydrolase activity has been associated with higher susceptibility to bleomycin and chronic fibrosis. , Genetic variations may play a role in determining sensitivity to lung toxicity of bleomycin as exemplified by studies that have shown variable susceptibility in murine strains with different genetic variants. Inflammatory response mediated by T cells and cytokines appears to be central to the pathogenesis, suggested by the finding that athymic mice appear to be resistant to bleomycin lung toxicity. The fact that soluble Fas antigen and anti-FasL antibodies have been successful in preventing the progression of bleomycin-induced pulmonary fibrosis supports the role of these in the pathogenesis of this entity. Animal models have demonstrated increased intrapulmonary TNF-α and IL-1β after bleomycin exposure. , Free radicals and oxidative damage also appear to play a role in bleomycin toxicity. In addition, bleomycin activates fibroblasts both directly and indirectly due to cytokines such as TNFα, leading to increased collagen deposition and fibrosis. This process is mediated at least in part by transforming growth factor β (TGF β).
- ■
Presentation: Patients often present with dyspnea, cough, or asymptomatic reduction in DLCO within 1 to 6 months of initiating therapy. Bleomycin has been reported to present with eosinophilic or hypersensitivity pneumonitis, interstitial pneumonitis, acute lung injury or ARDS, and even pulmonary veno-occlusive disease and, on rare occasions, delayed pulmonary manifestations in the form of pulmonary fibrosis (see Fig. 8.1 ). Additionally, some authors have reported episodes of chest pain during infusion of bleomycin which was self-limiting.
- ■
- ■
Mitomycin-C: Mitomycin-C is an antineoplastic antibiotic and a cell cycle specific alkylating agent derived from Streptomyces caespitosus. Due to availability of more efficacious regimens, the clinical utility of mitomycin-C in the United States is mostly limited to the treatment of anal cancer. An estimated 2% to 12% of patients exposed to this agent develop pulmonary toxicity. Toxicity associated with mitomycin-C increases with higher doses, especially above 20 mg/m 2 . , Other factors that may lead to a higher risk of lung toxicity include supplemental oxygen, other medications with pulmonary toxicity, and thoracic radiation. Acute bronchospasm, which is usually self-limited, can occur in less than 6% of patients. There appears to be an association between the risk of lung toxicity and coadministration of vinca alkaloids even weeks after receiving mitomycin-C. , A similar association with vinca alkaloids has also been noted in patients who develop acute lung injury or ARDS. Chronic interstitial pneumonitis and rarely pleural disease and pulmonary veno-occlusive disease have been noted.
- ■
Doxorubicin: Doxorubicin is an anthracycline antitumor antibiotic. It has widespread applications in multiple tumor types including breast cancer, acute lymphoblastic leukemia, Hodgkin’s lymphoma, and non-Hodgkin’s lymphoma, among others. Patients may notice dyspnea during the infusion of the pegylated liposomal doxorubicin. Other lung toxicities that have been reported include interstitial pneumonitis, organizing pneumonia, and radiation recall pneumonitis.
- ■
Epirubicin and mitoxantrone: Some topoisomerase II inhibitors such as epirubicin and mitoxantrone have also been associated with pneumonitis; however, the reported cases were usually also receiving concomitant chemotherapy with other agents that could have potentially contributed to the toxicity.
ALKYLATING AGENTS
- ■
Busulfan: Busulfan is commonly used for conditioning prior to stem cell transplantation. Prior to the availability of tyrosine kinase inhibitors, it was also used for the treatment of chronic myelogenous leukemia (CML). Overall, less than 8% of patients who receive the drug develop pulmonary toxicity. Busulfan is associated with increased risk of pulmonary complications because it is often coadministered with other drugs such as cyclophosphamide. Other risk factors include radiation exposure, prolonged exposure, or a cumulative dose above 500 mg. , A variety of histopathologic findings have been noted in patients with busulfan toxicity including degeneration of type 1 pneumocytes and atypical hyperplasia of type II pneumocytes, mononuclear cellular infiltrates, diffuse alveolar damage, and alveolar hemorrhage. Clinically, most lung toxicities manifest between 1 month to 1 year after stem cell transplantation and manifest as acute lung injury, chronic interstitial fibrosis, or alveolar hemorrhage.
- ■
Melphalan: Melphalan is also a drug used for stem cell transplantation conditioning. On rare occasions, acute bronchoconstriction and pneumonitis have been reported with melphalan use.
- ■
Cyclophosphamide: Although pulmonary toxicity from cyclophosphamide is rare (<1%), due to its widespread clinical application and potential for severe lung involvement, clinicians should be aware of the possibility of these toxicities. Some patients present with reversible pneumonitis within 1 to 6 months of receiving the drug, whereas others present with delayed pneumonitis or fibrosis even years after therapy. Late onset pneumonitis and fibrosis are usually resistant to treatment with steroids and eventually lead to terminal lung fibrosis. , Risk factors for these toxicities are increased FiO 2 exposure, radiation, and concomitant drugs with the potential for pulmonary toxicity.
- ■
Ifosfamide: Ifosfamide can cause pneumonitis similar to that observed with cyclophosphamide. Additionally, ifosfamide uniquely can cause methemoglobinemia. The mechanism for this is via a metabolite of ifosfamide which reacts with glutathione, resulting in the depletion of the red blood cell antioxidant reserve and leading to methemoglobinemia.
- ■
Chlorambucil: Chlorambucil is used mainly for the therapy of chronic lymphocytic leukemia. Pulmonary toxicity has been rarely observed with this drug but can occur during, or even after, discontinuation. In addition, there does not appear to be a correlation between the dose or duration of therapy. Patterns of lung involvement include chronic interstitial pneumonitis, pulmonary fibrosis, organizing pneumonia, and acute interstitial pneumonia.
ANTIMETABOLITES
- ■
Methotrexate: Methotrexate inhibits dihydrofolate reductase and leads to impaired DNA synthesis. Risk factors for pulmonary toxicity include age older than 60 years, involvement of the lungs or pleura with rheumatoid disease, prior therapy with disease-modifying antirheumatic drugs, and diabetes mellitus (hyperinsulinemia in treated diabetes can lead to increased polyglutamation of methotrexate). The most common pattern of toxicity observed is hypersensitivity pneumonitis, which usually occurs days to weeks into treatment, although delayed reactions have been observed. Other forms of toxicity include organizing pneumonia, pleural effusions, and pulmonary fibrosis ( Fig. 8.2 ). Methotrexate-associated pneumonitis has a few classic features that set it apart from other reactions—it is usually reversible, accompanied by mild eosinophilia in over half of the cases, associated with weakly formed granulomas in the lung, and has a low chance of recurrence with methotrexate rechallenge after resolution of symptoms. In addition, hilar lymphadenopathy can be seen in close to 10% of patients. Proposed mechanisms for toxicity include activation of the mitogen-activated protein kinase pathway, altered cytokine milieu, alveolar epithelial injury, and host reaction to latent viral infections, such as cytomegalovirus or Epstein-Barr virus. ,