Puberty is the transitional process leading to reproductive maturity. Proper diagnosis and management of pubertal disorders requires understanding of (1) basic endocrinology of the hypothalamic-pituitary-gonadal axis; (2) developmental changes that occur at different points throughout childhood; and (3) the wide variability in timing of physical changes of puberty seen in normal children.
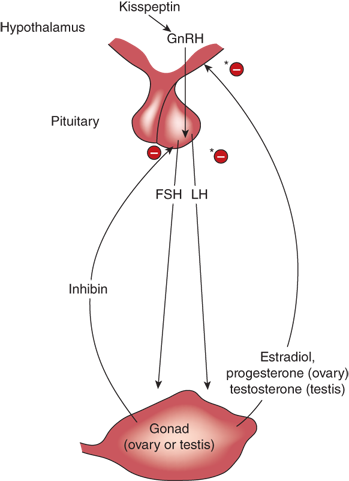
The three anatomic sites most pertinent to pubertal development are the hypothalamus, the anterior pituitary gland, and the gonads (ovaries or testes), known collectively as the hypothalamic-pituitary-gonadal (HPG) axis (Figure 3-1). Also shown in this figure are the critical hormones and feedback loops of the HPG axis.
Kisspeptins are peptides secreted by neurons in the arcuate and anteroventral periventricular nucleus of the hypothalamus.1 These peptides bind to a G-protein–coupled receptor called GPR54 on hypothalamic neurons that secrete gonadotropin-releasing hormone (GnRH). The resulting pulses of GnRH in turn induce pulsatile secretion of the gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH). LH and FSH working in concert lead to germ cell (spermatozoa or ova) maturation and also stimulation of sex steroid (androgen or estrogen) secretion by the gonads, along with gonadal peptides such as inhibins. The sex steroids exert a negative feedback effect at both hypothalamic and pituitary levels, reducing the secretion of kisspeptin, GnRH, and LH, whereas inhibins control FSH secretion via a separate negative feedback loop at the pituitary level. Later in life, once menstrual cycles begin, women also develop a positive feedback cycle whereby estrogens stimulate gonadotropin release during phases of the cycle critical for ovulation.
Inhibins are dimeric gonadal peptides that inhibit FSH secretion by the pituitary gland. The two major forms of inhibin are inhibin A and inhibin B. Inhibin A is a product of ovarian follicles and is only measurable in females. Inhibin B is the product of granulosa cells in girls and of Sertoli cells in boys, and may be useful, along with anti-müllerian hormone (AMH), for evaluation of testicular abnormalities, including cryptorchidism.
Activins are dimeric gonadal proteins that stimulate FSH secretion, although there appears to be less clinically relevant information gleaned from measuring activins than there is from measuring inhibins.
These basic hormonal pathways are modulated by many different central nervous system (CNS) inputs, including stimulatory effects of glutaminergic neural systems, inhibitory effects of γ-aminobutyric acid (GABA)-ergic systems, and direct interactions between glial cells and hypothalamic neurons. The CNS in turn integrates the effects of many external factors such as nutrient availability, exercise, stress, social/psychological factors, and indirect effects of chronic disease.
Four critical developmental steps during fetal life establish the required anatomy for the HPG axis and normal pubertal development. First, GnRH-producing neurons must migrate from the olfactory region to proper positions in the hypothalamus, with dependence on products of the KAL-1 (Kallmann syndrome 1) and FGFR1 (fibroblast growth factor receptor-1) genes for normal anatomical development. Second, the mechanism for pulsatile GnRH secretion and responsiveness must be established, with dependence on genes such as KISS-1 (producing kisspeptin) and GnRHR (GnRH receptor). Third, normal development of the hypothalamus and gonadotropin-secreting cells of the pituitary depends on genes such as NR5A1 (SF-1), NROB1 (DAX-1), PROP1, HESX1, LHX, LH-β, LHR (LH receptor), FSH-β, and FSHR (FSH receptor) among others.2 Fourth, the gonads must form properly, with differentiation of Leydig and Sertoli cells in the testis, and granulosa and theca cells in the ovary.
Provided that these developmental steps occur properly, the pubertal mechanism is already active for brief periods during fetal, neonatal, and early infant life (“mini-puberty of infancy”). Understanding when infants normally have elevated LH, FSH, and sex steroids (Figure 3-2) allows efficient laboratory assessment whenever the integrity of the HPG axis is in question, as with congenital or acquired CNS, pituitary, or gonadal abnormalities. After the first year of life, the pubertal mechanisms are quiescent, reflecting an active suppression of pulsatile GnRH secretion that remains in place until the child approaches the time of adolescence. During this period of pubertal latency, it is difficult to distinguish the child who has hypogonadotropic hypogonadism (pathologic inability to produce sex steroids owing to hypothalamic-pituitary abnormalities) from the normal child whose GnRH secretion is still under active restraint. In addition, the knowledge that the underlying pubertal mechanism is dormant only because of active CNS restraint helps explain why CNS abnormalities are often associated with precocious puberty.
Figure 3-2
Gonadal steroids during early childhood. Columns at left (girls yellow, boys green) indicate cord blood levels. Solid red line indicates levels in boys; dotted blue line indicates levels in girls. Note the marked testosterone peak (7 nmol/L is ~200 ng/dL) during the first few months of life in boys. A more subtle increase in estradiol occurs in infant girls before decreasing to prepubertal levels.

The harbinger of puberty is the increase in pulsatile release of GnRH, which occurs first at night, well over a year before any physical signs of puberty appear. Specific proteins that repress KISS-1 have recently been identified in rats, and it appears that silencing of the genes for these repressor proteins is the trigger for initiation of puberty. Though measurement of GnRH in peripheral blood in humans is of little clinical value for assessment of puberty, LH serves as a good proxy for the return of GnRH pulsatility, provided that an ultrasensitive LH assay (with analytical sensitivity down to 0.1 IU/L) is used. For most of these ultrasensitive assays, an LH level above 0.3 to 0.55 IU/L is suggestive of early central activation of puberty.
In boys, the rise in testosterone matches the increase in LH levels. The binding protein for both testosterone and estradiol, sex hormone–binding globulin (SHBG) declines as testosterone increases, leading to a rapid rise in the free (active) fraction of testosterone. Estrogen levels also increase somewhat in boys during puberty mostly from peripheral conversion (aromatization) of testosterone to estradiol. The peak of this estradiol rise is typically seen around the age of 13, corresponding to mid-puberty (Tanner stage 3—see as follows), the period during which adolescent male breast enlargement (gynecomastia) is most often seen.
In girls, estradiol secretion (in response to both FSH and LH) during early puberty is episodic, making random measurement of estradiol an insensitive marker for pubertal onset, especially compared to ultrasensitive assays for LH. Although rising levels of estradiol should theoretically increase SHBG levels in girls as they progress through puberty, what is actually observed is a mild downward trend in SHBG, likely caused by other factors known to decrease SHBG, such as rising insulin-like growth factor-1 (IGF-1), increase in average insulin levels during adolescence, and increased secretion of androgens by the adrenals and the ovaries.
Although often used as a synonym for pubarche, the first appearance of pubic hair, the term adrenarche is better defined biochemically as a detectable increase in the secretion of adrenal androgens, including androstenedione and dehydroepiandrosterone (DHEA) associated with a significant increase in CYP17A1 expression (resulting in activation of 17α-hydroxylase/17,20-lyase in the zona reticularis in the adrenal cortex) starting at age 5 years, or its longer-lived sulfated metabolite, DHEAS. These biochemical changes represent a gradual process that may start well before the age of 6, several years before pubic hair appears or puberty actually begins.
Growth hormone (GH) pulses increase in amplitude during puberty, with a resulting increase in circulating IGF-1 concentrations. These changes may explain the transient insulin resistance seen during early and mid-puberty, a phenomenon that is independent of any insulin resistance arising from increased body mass index (BMI) or total adipose tissue.
The end result of the developmental and endocrinologic changes described previously is the appearance of the physical changes that characterize the final phase of puberty. In girls, the most obvious changes involve breast development (thelarche), the presence of pubic hair (pubarche), and the onset of menstrual periods (menarche). For boys, the focus is on genital and pubic hair development as well as increase in muscle strength, deepening of the voice, and appearance of facial hair. Any delay in pubertal onset also delays the expected acceleration of growth, so that the very common referrals of adolescents for short stature and delayed growth also requires assessment of pubertal status.
All guidelines for pubertal norms should be viewed with circumspection, as studies of puberty in children are fraught with major methodological (issues with design or measurement) and statistical difficulties.3,4 Therefore, though a study may demonstrate convincingly that breast development in a 7-year-old girl can be normal, such a study cannot be used to predict that breast development in a specific 7-year-old patient is normal. More information from medical history and physical examination, and perhaps biochemical evaluation is required, as well as monitoring the rate of progression of the breast development.
Family studies, particularly twin studies, suggest that genetic factors contribute to at least one-half to three-quarters of the variability in timing of puberty.2,5 Ethnicity is a known factor, with black girls showing earlier onset of breast development and especially adrenarche compared to white girls. Table 3-1 lists many of the genes associated with normal puberty or with pubertal disorders.
| Gene | Gene Product | Pubertal Disorder Associated with Gene Defect |
|---|---|---|
| FGFR1 | Fibroblast growth factor receptor 1 | Kallmann syndrome |
| FSH-β | FSH-β subunit | Idiopathic hypogonadotropic hypogonadism |
| FSHR | FSH receptor | Idiopathic hypogonadotropic hypogonadism |
| GNAS | Gsα protein | Precocious puberty (activating mutation) in McCune-Albright syndrome |
| GNRHR | GnRH receptor | Idiopathic hypogonadotropic hypogonadism |
| KISS-1 | Kisspeptin | Precocious puberty and idiopathic hypogonadotropic hypogonadism |
| GPR54 | Kisspeptin receptor | Precocious puberty and idiopathic hypogonadotropic hypogonadism |
| HESX-1 | “Homeobox gene expressed in ES cells” HESX1 protein | Abnormal hypothalamic-pituitary development |
| KAL1 | Anosmin-1 | Kallmann syndrome |
| LEP | Leptin | |
| LEPR | Leptin receptor | Idiopathic hypogonadotropic hypogonadism and obesity |
| LH-β | LH-β subunit | Idiopathic hypogonadotropic hypogonadism |
| LHR | LH receptor | Idiopathic hypogonadotropic hypogonadism (inactivatingmutation); ”testotoxicosis” (activating mutation) |
| LHX3 | “LIM family of homeobox transcription factors” | Abnormal hypothalamic-pituitary development |
| NELF | Nasal embryonic luteinizing hormone–releasing hormone factor | Kallmann syndrome |
| NR5A1 (SF-1) | Nuclear receptor, subfamily 5, group A, member 1 (steroidogenic factor-1) | Abnormal hypothalamic-pituitary development, adrenalhypoplasia |
| NR0B1 (DAX-1) | Nuclear receptor, subfamily 0, group B, member 1(dosage-sensitive sex reversal—adrenalhypoplasia congenital critical region on the X chromosome) | Abnormal hypothalamic-pituitary development, adrenalhypoplasia |
| PC1 | Prohormone convertase-1 | Idiopathic hypogonadotropic hypogonadism andhypoglycemia |
| PROK2 | Prokineticin-2 | Kallmann syndrome |
| PROKR2 | Prokineticin receptor 2 | Kallmann syndrome |
| PROP1 | “Prophet of Pit-1” | Abnormal hypothalamic-pituitary development |
A girl’s nutritional status, as assessed by BMI and/or measurement of body fat mass, is strongly associated with alterations in pubertal timing.6 Girls who are significantly underweight or who have extremely low body fat are more likely to have delayed menarche and/or secondary amenorrhea. Higher BMI/obesity in girls is associated with earlier appearance of breasts, pubic hair, and onset of menstrual periods, with evidence pointing to leptin, a product of adipose tissue, as a signal that must reach a minimum threshold for the initiation of puberty.7 Based on these findings and arguments from evolutionary biology, the current hypothesis is that increased body fat is a cause of earlier puberty in girls, although alternate hypotheses (eg, increased fat is the result, rather than the cause of, an accelerated pubertal process) may still be possible.
The link between higher body fat and altered timing of puberty is less obvious in boys, which may either reflect different evolutionary pressures or simply the greater methodological difficulty of scoring male genital development (as opposed to noting onset of menarche in girls) for studies of puberty in boys. The trend appears to be that higher BMI in boys is associated with later, rather than earlier, onset of puberty.
The effect of environmental chemical exposures on the timing of puberty is a topic under great debate and active research.8 A wide variety of endocrine-disrupting chemicals have been identified that have the ability to mimic (agonists) or block (antagonists) the effects of sex steroids, or have toxic effects on critical components of the reproductive axis. Examples include pollutants such as polychlorinated or polybrominated biphenyls, dioxins, pesticide or fungicide residues, phthalates and other compounds from plastics, toxins such as lead, or even naturally occurring food components such as soy isoflavones. Further complicating the story is that endocrine-disrupting chemicals may exert their effects during the prenatal or prepubertal years as well as in the immediate peripubertal period, making epidemiological study of these compounds an extremely ambitious task. While awaiting results of further studies, the clinician can provide some reassurance to anxious parents that these compounds are generally two or more orders of magnitude weaker than primary sex steroids like estradiol. Accordingly, avoidance of obvious sources of known endocrine disruptors would becommon sense.
Typically, significant chronic disease in childhood is associated with a later onset of puberty. Exceptions include diseases involving the CNS, where there may be disturbances of the normal mechanisms that restrain puberty during the childhood years. In some cases, the effects are mixed, as with cerebral palsy, where onset of breast development may occur earlier, despite later onset of menstrual periods.
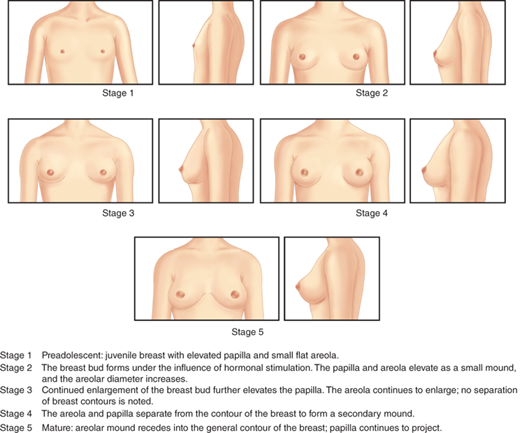
Thelarche is the onset of breast enlargement in girls. Asymmetrical breast development, with one breast developing before the other, or a mild difference in sizebetween the two breasts, is not uncommon. Criteria for Tanner staging of breast development are shown inFigure 3-3. A common difficulty in staging of breast development is encountered with overweight girls, where even a physician may not be able to distinguish by observation alone between fatty tissue in the breast area versus actual glandular tissue. Palpation of breast tissue is a more accurate method for determining if Tanner stage B2 has truly been attained. Other issues include the lack of an absolute objective boundary between stage B2 and B3, as well as determining the difference between a large breast at stage B3 and a small breast at stage B5. In the latter case, a more developed papilla (the pigmented projection of the nipple) close to 1 cm in diameter suggests stage B5.
A number of recent studies suggest that the mean age at thelarche is somewhere between 9 and 10 years in white or Asian girls, several months earlier in black girls, and somewhere in between for Hispanic girls.9, 10, and 11 Mean age, however, is not particularly helpful when faced with patients referred for evaluation of precocious or delayed puberty. The lower age threshold for breast development in healthy girls appears to be earlier than in previous decades, with some pediatric endocrinologists in the United States subscribing to an age threshold of 7 years in white girls and 6 years in black girls.12 Additional support for this threshold comes from a study of over 1200 girls in three US cities, where 10% of white girls, 14.9% of Hispanic girls, and 23.4% of black girls had reached breast Tanner stage II before 8 years of age.13 In clinical practice, however, many pediatric endocrinologists continue to be cautious about white girls who start puberty before 8 years (or black girls before age7 years), preferring to evaluate such girls carefully before labeling their puberty as normal.3 At the upper end, the +2 standard deviation (SD) age threshold for breast development is just under 13 years of age. Regardless of which cutoffs are used, statistically derived age thresholds should never be used as absolute indicators of health or disease without additional clinical and biochemical information.
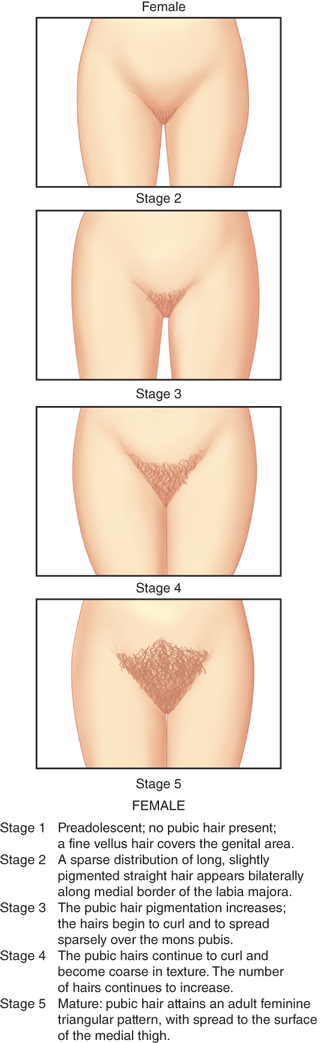
Pubarche is the first appearance of pubic or axillary hair, although typically axillary hair appears 1 to 2 years after pubic hair in white girls (this time gap may be shorter in black girls). Because pubarche is so dependent on adrenal androgen secretion, the terms pubarche and adrenarche are frequently used interchangeably, although, as noted previously, the onset of increased adrenal androgen secretion may actually precede pubarche by a few years. Although pubarche typically occurs around the same time as gonadarche (activation of the HPG axis), each is actually the result of different regulatory pathways (adrenal vs gonadal) and may, on occasion, follow significantly different timing patterns. Therefore, the appearance of pubic hair should not be considered a sign of true pubertal onset.
Criteria for Tanner staging of pubic hair development are shown in Figure 3-4. In some ethnic groups, the amount of pubic hair is less for any given Tanner stage. There is no Tanner staging scale for axillary hair, which is rated on a simple three-point scale, where stage 1 signifies no hair, stage 3 signifies axillary hair adult in quantity and quality, and stage 2 is somewhere in between.
From available studies, the median age of pubarche is 10.5 to 10.9 years in white girls, 8.8 to 9.4 years in black girls, and around 10.4 years in Hispanic girls. Estimates of the lower age limit for pubarche vary widely among different studies, ranging from 8 to 9 years in white girls. Black girls have a significantly earlier pubarche, with at least one study noting that 5.2% of 4-year-old healthy black girls already had some pubic hair.
Menarche is the onset of menstruation in girls. The mean age of menarche is about 12.6 years in white girls, 12.1 years in black girls, and 12.2 years in Hispanic girls. The lower (~ −2 SD) age threshold is about 10.5 years in white girls and 10 years in black girls. The upper (+2 SD) cutoff is between 14.5 and 15 years for white girls and about 14 and 14.5 years in black girls.
Of note, girls who have earlier thelarche may have a slightly longer period of time before menarche. Thus, a girl with thelarche at the age of 9 years may have 2.8 years before menarche, while another girl with thelarche at the age of 11 years may only have 1.8 years until menarche. There are also girls who have early thelarche, but whose tempo of pubertal progression is slow, with a normal final outcome; thus, the tempo of puberty should be part of the assessment of pubertal development.
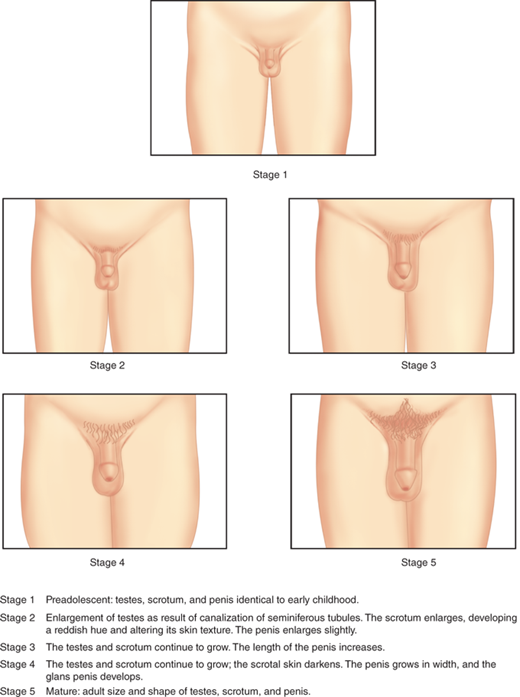
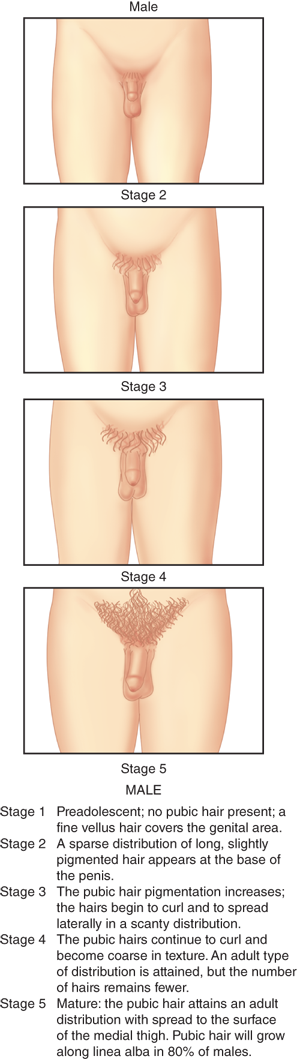
Although there exists a system for Tanner staging of male genital development during puberty (Figure 3-5), in practice, the differences among the intermediate stages are too subjective for consistency from observer to observer. Much more accurate is the measurement of testicular size using a model such as the Prader orchidometer, shown in Figure 3-6. A volume of 3 to 4 mL is considered to be an indicator of pubertal onset, as the seminiferous tubular volume increases under gonadotropin stimulation. If an orchidometer is not available, a testis length of 2.5 cm along the longest axis corresponds to approximately 4 mL volume. The median age at testicular enlargement to 3 mL volume, based on a recent study of over 4000 subjects, is 10.14 years in white boys and 9.14 years in black boys.14 While these data suggest that testicular enlargement to 3 mL can be normal in boys as young as 7 years, many pediatric endocrinologists continue to evaluate closely any boys who start puberty before age 8 years. Lack of testicular enlargement by the age of 14 years is generally considered to be delayed.
Tanner staging criteria for pubic hair in boys are shown in Figure 3-7. As with girls, androgen secretion from the adrenals is an important determinant of pubarche. However, androgen secretion from the testes is also a major factor in pubarche, increasing the chances that precocious pubic hair development in boys may signify true precocious puberty.
The average age of pubarche in boys is around 12 to 12.5 years in white and Hispanic boys, with black boys starting about 1 year earlier. Pubarche before the age of 10 years is commonly considered worthy of further observation and clinical workup, while lack of pubic hair development after the age of 14.5 years in white and Hispanic males (after 14 years in blacks) can be considered delayed. Axillary hair typically appears about 1 to 2 years after pubic hair.
Spermarche, the ability to produce and ejaculate sperm, typically occurs around the age of 13.5 years (at least Tanner stage 3). Voice break, the transition to the deeper adult voice, occurs at a mean age of about 13.9 years. Peak height velocity, increased muscular strength, and worsening of acne typically occur around this same time.
Facial hair, usually starting with the upper lip, typically appears around the age of 15 years, but may not be of full adult quality until well past the teenage years.
Girls have their peak growth velocity early in puberty, typically beginning right before thelarche. Boys, in contrast, have their peak growth spurt during mid-puberty (between Tanner stages 3 and 4), at a mean age of 13.5 years, often corresponding to a testicular volume of 10 to 12 mL.
A common misconception is that the amount of growth remaining for a girl after menarche is only a couple of inches, which often leads to predictions of severe short stature in girls who have early normal menstruation.15 In fact, the amount of growth remaining varies greatly depending on when menarche occurs. Thus, a girl who has menarche at the age of 10 years grows, on average, an additional 4 in, whereas another girl who has menarche at the average of 12.7 years will grow on average another 3 in. The girl who has menarche at the age of 15 years may only grow 2 in more, but because she is already at a taller height by the time that she has her first menstrual period, her final height is likely to be within her expected genetic potential.
The first sign of clinically detectable puberty in girls is breast development in 85% of cases with pubic hair preceding thelarche in the other 15%. In actuality, ovarian enlargement is the first true sign of female puberty, but it requires ultrasonography for detection. Analogously, testicular enlargement is the first sign in boys. Whether puberty is truly occurring earlier than was previously thought, especially in girls, is controversial as some of the suggestive large-scale epidemiological data (ie, the Pediatric Research in Office Settings [PROS] network9) may be flawed because of ascertainment bias in subject selection, use of visual (as opposed to manual) determination of breast staging, and failure to perform diagnostic testing in children in the younger age ranges to definitively rule out pathological causes of precocity. A new report on the timing of male pubertal onset also from the PROS network suggests that genital and pubic hair growth and testicular enlargement are occurring 6 months to 2 years earlier than in past studies, especially in blacks.14 That said, Danish Registry data suggest that 0.2% of all Danish girls and fewer than 0.05% of all Danish boys have some sort of precocious puberty.
Thus, the clinician must first determine in a child with “borderline” early puberty whether observed changes may represent normal variants or have a more serious organic basis. The next dilemma is when and if diagnostic testing is required as often nothing more than a bone age is required in clear examples of normal or slowly progressive variants. This decision may often be best left to the pediatric endocrinology consultant who can, in the most cost-effective manner, determine whether, how (basally or after stimulation), where (noting tremendous variability in assays and clinical laboratories), and how often testing is required. Finally, decisions regarding the necessity for and type of treatment will usually require the specialist’s input, noting that not all organic etiologies and certainly not the normal variants require treatment.
Most commonly, premature thelarche begins around 2 years of age, but it may be present from birth onward (Figure 3-8A). The observed tissue may be asymmetrical, unilateral, or bilateral. Although asymmetrical and/or unilateral breast tissue often causes parents to be concerned about the possibility of malignancy, this is rarely the case. In 30% to 60% of affected girls, the early breast tissue spontaneously regresses within an average of 18 months from its development. When it persists, the degree of breast development typically does not exceed Tanner stage 3.
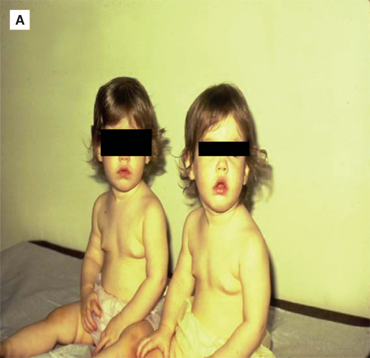
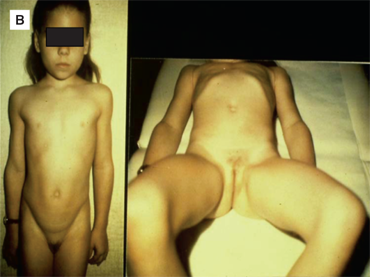
Figure 3-8
(A) Twin 2-year-old girls with idiopathic precocious thelarche and no other signs of puberty. (B) 5-year-old girl with idiopathic precocious adrenarche and no other signs of puberty. (C) 3-year-old girl (right) with idiopathic isosexual central precocious puberty characterized by both breast and pubic hair development, along with tall stature, contrasted to a normal 5-year-old prepubertal girl (left). (A and Creproduced from: Geffner ME. Disorders of puberty. In: Kliegman RM, Greenbaum LA, Lye PS, eds. Practical Strategies in Pediatric Diagnosis and Therapy. Harcourt Health Sciences; 2005; with permission of Elsevier.)

The etiology of typical isolated premature thelarche is unknown. Most studies have failed to show differences between basal and GnRH-stimulated gonadotropin levels in girls with simple premature thelarche versus age-matched normal girls (both of whom has an FSH-predominant response); in contrast, girls with true precocious puberty have an LH-predominant response. Conventional assays usually cannot detect elevated serum estradiol levels in girls with isolated premature thelarche, although higher mean levels compared to those in age-matched normal girls have been reported using an ultrasensitive recombinant cell bioassay, but with significant overlap between the two groups. By default, heightened sensitivity of breast tissue to estrogen has been invoked as a potential etiology. A causative role for environmental endocrine disruptors has also been suggested. A strong association between the prevalence of premature thelarche and BMI has recently been reported in 4- to 8-year-old girls. In cases of either exaggerated isolated thelarche with advanced bone age and/or increased height velocity or self-limited recurrent episodes of isolated breast-budding that may be associated with advanced bone age and/or stepped-up height velocity, activating mutations of the Gsα gene have been reported in up to 25% of affected girls, akin to the mutation reported in full-blown McCune-Albright syndrome (see section Idiopathic Isolated Precocious Menarche).
For girls with “classic” precocious isolated thelarche, that is, starting at or prior to the age of 2 years, who, by definition, have no associated growth spurt and a bone age that is within 2 SD of their chronological age, no other diagnostic studies typically need to be performed. Such girls usually will go on to have menarche at a normal age and attain normal adult stature. Because a small subset of girls who present with precocious thelarche may progress to either a variant characterized by isolated progressive breast development in conjunction with accelerated linear growth and advancing bone age or to true precocious puberty, ongoing follow-up either by a pediatrician or a pediatric endocrinologist is mandatory with judicious use of additional/ongoing laboratory and radiologic testing as dictated by the clinical course. The presence of a mild degree of thelarche, without androgenic manifestations of puberty, may also occur in the very earliest stages of peripheral precocious puberty caused by a granulosa cell tumor.
Unless the condition progresses as noted earlier, no treatment is indicated.
This is another common pubertal variant that is characterized by the early development of pubic hair, axillary hair and odor, and/or a small amount of acne using the same age criteria defined previously, along with elevation of adrenal-specific steroids, such as DHEA and/or DHEAS; without this biochemical confirmation, the entity should just be called precocious pubarche (Figure 3-8A).
Premature adrenarche appears to result from increased production of adrenal androgens in both sexes at an earlier than normal age resulting from premature maturation of the zona reticularis of the adrenal cortex.16 More specifically, it has been proposed that there is a reduction in 3β-hydroxysteroid dehydrogenase activity and induction of the 17,20-lyase component of the P450c17 enzyme complex in the zona reticularis through preferential hyperphosphorylation secondary to an autosomal dominant mutation of the kinase responsible for serine/threonine phosphorylation, leading to increased serum concentrations of DHEA and DHEAS. Some children with precocious adrenarche have been reported to have heightened androgen receptor gene activity whereas others have been found to be more likely to harbor a specific polymorphism in the adrenocorticotropic hormone (ACTH) (melanocortin 2 receptor = MC2R) receptor promoter. Finally, etiologic or permissive roles for corticotropin-releasing hormone, insulin, IGF-1, and leptin have also been proposed. Precocious adrenarche occurs much more commonly in girls than in boys (perhaps as much as 10-fold) and appears to develop more often in obese and/or African American girls. It also appears to occur more often in children with low birth weight.
In both girls and boys, by definition, there is no clinical evidence of virilization, that is, no growth spurt, increase in muscle bulk, voice-deepening, or temporal hair recession (male pattern baldness). In addition, affected girls will not manifest clitoromegaly and boys will not have penile enlargement. Furthermore, there is no evidence of ovarian estrogen-mediated components of puberty in girls and no testicular enlargement or function in boys. If a child presents at a very young age, it is generally presumed that an organic cause is more likely to be found. However, in infant boys with isolated scrotal hair, typically no cause is found and the hair subsequently falls out within 12 months. In most cases of idiopathic precocious adrenarche, serum levels of DHEA and/or DHEAS are in the respective pubertal ranges for girls and boys, and the bone age is mildly, but not significantly, advanced. In girls with precocious adrenarche, serum levels of other adrenal steroids are usually normal or only slightly elevated for age, especially if measured after stimulation with ACTH, but normal after leuprolide administration. These observations suggest that precocious adrenarche in girls is associated with functional adrenal hyperandrogenism, but no biochemical evidence of ovarian hyperandrogenism. Thus, if a child presents with the classical phenotype, no other laboratory or radiological studies are usually warranted, unless there is significant rapidity of progression of symptoms. On rare occasion, children with more serious organic pathologies associated with hyperandrogenism (eg, nonclassic adrenal hyperplasia [NCAH], adrenal tumors, and gonadal tumors) or true precocious puberty may initially present similarly to those with apparent idiopathic isolated premature adrenarche. Thus, in unclear situations, serum measurements (at 8 am) of 17-hydroxyprogesterone (basally or after ACTH stimulation) and testosterone, along with ultrasounds of the adrenals and/or gonads, may need to be done. With the presence of isolated adrenarche in girls, measurement of LH, FSH, and estradiol is not indicated (as these should only be determined when there are isolated female changes of puberty or both female and male changes). Historically, this pubertalvariant has been considered to be benign and self-limited, but recent epidemiological and biochemical data suggest that, at least in girls with associated low birth weight and rapid postnatal catch-up growth, precocious adrenarche may be followed by early onset and rapid progression of true puberty as well as by future development of functional ovarian hyperandrogenism/polycystic ovarian syndrome (PCOS). Additional features of this syndrome include early-onset insulin resistance with a disturbance of the insulin-IGF-1 system, atherogenic lipid profiles, and hypertension (collectively known as the metabolic syndrome or syndrome X), and a positive family history of type 2 diabetes mellitus (T2DM), hypertension, PCOS, and anovulation beginning in late adolescence. Recently, it has been shown that boys with precocious adrenarche are also insulin-resistant. Thus, the adjective “benign” or term “normal variant” should not necessarily be applied to cases of premature adrenarche.
Currently, there remains no proven treatment for idiopathic precocious adrenarche and no specific means by which to forestall future functional ovarian hyperandrogenism, although basic practices to limit weight gain, regulate cholesterol intake, and avoid smoking seem advisable. Investigative use of metformin in children with precocious adrenarche has been shown, in small cohorts, to improve body composition and delay the onset of true puberty, and, in so doing, perhaps improve adult height.
This is defined as menstruation without any other manifestations of puberty in a young girl prior to the age of 9 years.
The cause is unknown, with the ultimate assignment of an idiopathic basis for its basis being a diagnosis of exclusion, so that one must also consider a foreign body, local masses, severe primary hypothyroidism, and McCune-Albright syndrome.
In the setting of isolated early menarche, the situation is usually self-limited, albeit disturbing to parents and patients. In most cases, there is only one period, but there may be two or more. Adult height and subsequent menstrual patterns and fertility are usually normal.
None is required.
No formal definition exists.17 By the use of a statistical approach, pubertal progression may be considered unduly fast if the start of puberty is within the normal range for gender and race, but subsequent milestones are achieved more than 2 SD ahead of their expected mean age of occurrence. As guidelines, in girls, the typical interval from breast budding (Tanner stage 2)to menarche (usually associated with at least Tanner stage 4 breasts) is 2.4 ± 1.1 years (mean ± 1 SD). In boys, the usual time from pubertal onset (Tanner stage 2 testes, ie, ≥ 3 mL in volume) to adult testicular volume (mean 20 mL) is 3.2 ± 1.8 years.
The exact etiology is unknown. Factors that may modify the rate of pubertal maturation include family genetics, a history of low birth weight (small for gestational age [SGA]), rapid and/or excessive growth in early childhood (especially if having been born SGA), diet, diminished physical activity, and an array of other factors (eg, treatment of hypothyroidism, adoption, and endocrine disruptors).
Unfortunately, from a practical standpoint, the typically affected adolescent usually presents to medical attention nearly or even fully developed and having had marked height acceleration unduly early. In fact, such adolescents may already be in their postspurt height deceleration phase as a result of markedly advanced bone age that appears as a by-product of the phenomenon without yielding the requisite number of inches that would have occurred with a normal pubertal tempo. This process is often missed in a first affected child in a family because of the natural infrequency of visits to the primary care provider for well-child physical examinations in this age group.
There are also no proven treatment strategies for rapid tempo puberty. In fact, when identified late as is often the situation in the index case in a family, there is often no viable treatment option. When there is reasonable remaining growth potential, transient interruption of the HPG axis, as is done in children with bona fide central precocious puberty, is usually considered using GnRH analogs without or with added GH. However, in the United States, use of either drug class to treat rapid tempo puberty is not FDA-sanctioned, so that their use may be moot because of an inability to obtain insurance company authorization. More importantly, there are no data demonstrating treatment efficacy in this situation, although, from a theoretical standpoint, combination therapy might be helpful. Use of other off-label agents, such as aromatase inhibitors in boys, may be considered, but once again this approach is based solely on a theoretical basis and anecdotal experience.18
Central precocious precocity (CPP) results from activation of the HPG axis at an earlier-than-normal age (Figure 3-8A).19 Although as noted previously that the start of normal puberty may be occurring earlier than previously thought, most pediatric endocrinologists still consider 8 years in girls and 9 years in boys as general cut-points between early normal and early puberty, recognizing that an individualized approach needs to be taken for African American girls between 6 and 8 years, Caucasian girls between 7 and 8 years, and all boys between 8 and 9 years (as pathological causes of precocity have occasionally been reported in this age range), and especially in those children who present with a significant degree and/or rapid progression of puberty during these intervals. Isosexual development refers to pubertal changes appropriate for the sex of the child, such as breast budding in girls and testicular enlargement in boys. This contrasts with contrasexual development in which the pubertal features in females are male-hormone–directed, for example, clitoromegaly, or in males are female-hormone–directed, for example, breast development. In general, the presence of both female- and male-hormone–mediated features of puberty in females is a valuable clue to a central source for the precocity, although isolated estrogenic manifestations can occur as the initial presentation of precocious puberty, at least early on, in girls with a central etiology for their precocity. Additionally, on rare occasions, tumorous production of androgen by an adrenal tumor can provide sufficient substrate for either peripheral or intratumoralaromatization to estrogen, thereby confusing the clinical picture.
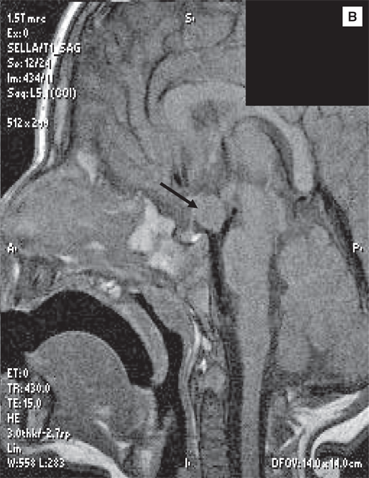
In females, most cases (~80%) of CPP have been considered to be idiopathic. With the application of magnetic resonance imaging (MRI), a somewhat higher percentage of organic causes has been detected in girls (Table 3-2). In contrast, only about 10% of central cases are idiopathic in males, with the remainder being associated with an underlying lesion of the CNS. The most concerning causes of CPP are tumors that arise in the suprasellar region, which may also be present with other, usually anterior, pituitary hormone deficiencies. Tumor-associated isolated CPP can occur with histologically benign hypothalamic hamartomas, the most common cause of CPP in very young children (Figure 3-9A and 3-9B). They may trigger puberty through secretion of GnRH (“ectopic GnRH pulse generator”) or transforming growth factor-β, another puberty-inducing growth factor. Hamartomas may, on occasion, be associated with gelastic (laughing) or other seizures. Other brain tumors that may cause CPP (with or without other pituitary hormone abnormalities) are glial cell tumors, germ cell tumors, and, occasionally, craniopharyngiomas, and pinealomas. The occurrence of combined “idiopathic” isolated GH deficiency or multiple anterior pituitary hormone deficiencies, an ectopic neurohypophysis, and CPP has been described.
|
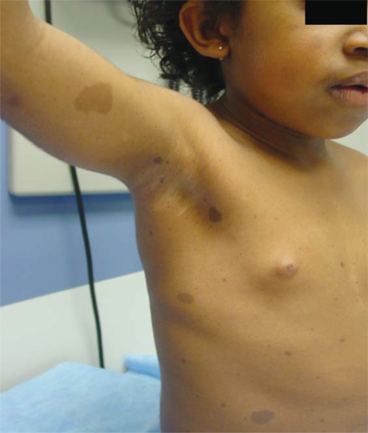
Other CNS causes include congenital brain defects that arise in the suprasellar region (eg, subarachnoid cysts, arachnoidoceles, and Rathke cleft cysts); other congenital midline anomalies (eg, hydrocephalus, meningomyelocele, and optic nerve hypoplasia [ONH]); cranial irradiation; previous meningoencephalitis; moderate-to-severe head trauma; and perinatal insult. The well-known association of CPP with neurofibromatosis-1 (Figure 3-10), characterized by associated smooth-bordered café-au-lait spots (resembling the coast-of-California), axillary and inguinal freckles, Lisch nodules, and neurofibromas, may result from the presence of a hypothalamic optic glioma, but may also have no detectable tumor on MRI. CPP may also occur in the setting of untreated or undertreated peripheral causes of puberty (see section Precocious Pseudopuberty), such as CAH, or after transdermal testosterone exposure, in which premature activation of the GnRH pulse generator occurs, presumably as a result of prior or ongoing CNS exposure to high levels of androgens (or androgens aromatized to estrogens). Another cause of sexual precocity that begins outside the HPG axis is referred to as the “overlap” or Van Wyk-Grumbach syndrome, in which CPP develops in the setting of long-standing untreated primary hypothyroidism. The mechanism of this phenomenon is not well understood, although, in the past, it had been ascribed to both elevated thyrotropin-releasing hormone (TRH) levels that stimulate pituitary FSH/LH secretion and, more recently, to the hypothyroid state itself in which the elevated TSH promiscuously interacts with gonadotropin receptors in the gonads. Rarely, males with hyperprolactinemia may develop CPP (although hypogonadism is much more likely). A heightened risk of CPP has been reported in children adopted from abroad, although this association may be clouded by inaccurate birthdates.
Figure 3-10
Four-year-old girl with neurofibromatosis demonstrating characteristic smooth-bordered (“coast-of-California”) café-au-lait spots, axillary freckles, and Tanner stage 3 breast development. (Photograph used with permission of Erica Eugster, MD [Indiana University School of Medicine, Riley Hospital for Children]).
Rare cases of CPP have been described in association with activating mutations of KISS-1, the gene encoding kisspeptin, as well as GPR54, the G-protein–coupled receptor mediating the effects of kisspeptin, the downstream effects of which, in either case, result in premature stimulation of GnRH neurons.20 The frequency of such mutations is unknown, but it should be pointed out that there is a 27.5% prevalence of familial CPP. At the current time, there is not enough phenotypic information to make specific recommendations as to who should be considered for genetic testing that is currently only available in research laboratories. In addition, serum kisspeptin levels have been reported to be useful marker of CPP. More recently, in some but not all populations, precocious puberty (and adult height) has been associated with certain haplotypes of the gene, LIN28B. This gene is related to both cell pluripotency and cancer, but how it might regulate pubertal timing is unknown.
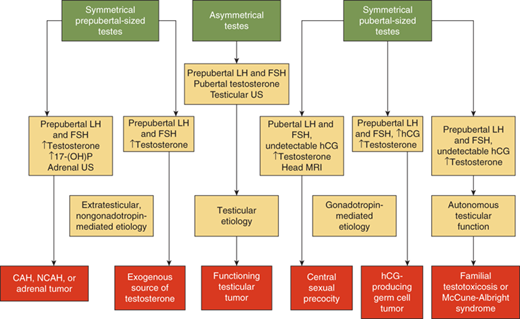
The initial evaluation of the child with precocious puberty is aimed at determining whether the process is normal or abnormal, has an idiopathic or pathological basis, what the rate of progression of the pubertal changes has been or will be, and whether the process originates centrally or peripherally (Figures 3-11 and 3-12). The initial test to perform is a bone age x-ray that provides an index of the potency of the process, although it is not useful in delineating the source of the hormonal process. More specifically, if not significantly advanced (ie, within 20% of the chronological age in months) and not associated with acceleration in height velocity, this suggests either a normal variant, slow progression pattern, or a process of relatively short duration. If a girl with precocious puberty has a significantly advanced bone age, a laboratory workup must be initiated that is dictated by the findings on the physical examination. Measurement of free T4 and TSH in both sexes is useful to rule out long-standing primary hypothyroidism that is usually apparent on clinical grounds.
If breast development is evident with or without androgen effects, measurement of random serum gonadotropins, using ultrasensitive assays for FSH and LH, and estradiol (using liquid chromatography tandem mass spectrometry or other methods to remove cross-reacting steroids) should be undertaken. Ultrasensitive bioassays for serum estrogenic bioactivity are under development. A pelvic ultrasound in this setting is a useful adjunctive diagnostic tool.21 Noting that mean prepubertal ovarian volumes (calculated for an oblate ovoid by multiplying the measurements in millimeters of the three dimensions by 0.523) increase gradually with age (3 years: 0.51 mm3;5.0 years: 0.78 mm3; 7 years: 1.14 mm3; and 9 years:1.31 mm3), the presence of bilaterally enlarged ovaries for age suggests hypothalamic-pituitary activation (or, less likely, McCune-Albright syndrome). In addition, the maturation of the uterus can be determined simultaneously and is useful as another indicator of the potency of estrogenic action. Measurement of serum androgen levels in girls with breast development with or without concomitant androgen effects rarely adds any useful diagnostic information. Because of the pulsatile and initially preferential sleep-entrained secretion of serum gonadotropins, random daytime measurements of LH and FSH may be low in the setting of central activation. However, use of immunofluorometric (IFMA), electrochemiluminescent (ECL), and high-sensitivity immunochemiluminescent (ICMA) assays for LH correlates much better with true CPP. However, if random gonadotropin levels are low, yet clinical suspicion suggests CPP, a GnRH stimulation test would next be performed.22 The most common protocol currently used for this test involves the subcutaneous administration of leuprolide acetate (not the depot form) at a dose of 20 μg/kg with measurement of gonadotropins every 30 minutes for 2 hours (usual maximum occurs at 30 minutes) and the sex steroid of interest 2 and 24 hours later. The results of these tests correlate remarkably well with those from multiple-sample, longer GnRH stimulation tests. The rationale for this interpretation is that, with central precocity, as with normal puberty, endogenous hypothalamic GnRH is being produced in a pubertal pattern that “primes” the pituitary gonadotrophs, so that, after administration of a single pharmacological dose of GnRH, there is abundant release of LH as typically occurs in teenagers. Measuring FSH levels during the test serves only to ensure biological activity of the leuprolide, and the peak concentration attained tends to be overlap between the prepubertal and pubertal state. Regardless, if CPP is confirmed biochemically and is progressive, a head MRI—in a closed unit—with contrast (required for detailed assessment of hypothalamic-pituitary region anatomy) must be performed to look for potential lesions in the hypothalamic-pituitary region, which are typically found in approximately 10% of girls with CPP. In cases of idiopathic CPP in girls, the MRI will frequently demonstrate exaggerated pituitary height (mean 6.2 ± 0.2 mm) as is typically seen in the adolescent female pubertal range.
The workup for a boy with isosexual precocity and a significantly advanced bone age is directed by the size of the testes. In cases of CPP, both testes are symmetrically enlarged for age, the degree to which will be governed by the potency and duration of the underlying etiology. Laboratory testing and the use of the leuprolide GnRH test are similar to that outlined above for girls, except for the substitution of testosterone for estradiol measurements. As with girls, if central precocity is confirmed biochemically, a contrast-enhanced head MRI must be performed as up to 50% of affected males will have demonstrable lesions of the CNS.
As to general issues related to treatment of precocious puberty, not all cases, including not all cases of CPP, require treatment (Table 3-3).23,24 Children with CPP or early normal puberty with a strong familial basis may profit from a period of observation without treatment, especially if outcomes of affected family members have been unremarkable. In addition, not all children with de novo CPP require treatment as a significant number have either a slowly progressive and/or transient process. Unless clinical findings suggest a rapid progression and/or significant resultant psychosocial difficulties, most children with CPP should be observed for a 3- to 6-month period before a decision to initiate GnRH agonist therapy is made.
Goal: inhibit secretion of gonadotropins and thereby reduce production of sex steroids by administration of GnRH analogs Leuprolide acetate (Lupron Depot-AbbVie) given as a monthly or 3-monthly intramuscular injection Histrelin (Supprelin–Endo) as a 12-mo subdermal implant Nafarelin acetate (Synarel-Syntex) given twice daily byintranasal route (rarely used) Leuprolide acetate (Lupron-AbbVie) given as a dailysubcutaneous injection (rarely used) |
The specific reasons that favor treatment include preservation of acceptable final stature based on family height genetics; prevention of psychological trauma (eg, from menstruation at a young age); reversal of mature physical appearance and risk of pregnancy in girls (as others assume that affected children are older than they appear); and reduction of anxiety, aggressiveness, and preoccupation with sexuality. Of note, serious psychological effects of early puberty are not typically encountered and GnRH agonist treatment typically only halts, but not actually reverses, existing physical changes of puberty induced by gonadal steroids. Thus, the goal of therapy is to inhibit secretion of gonadotropins and thereby reduce production of sex-specific sex steroids. This is accomplished by the administration of GnRH agonists that behave antagonistically because of biochemical modifications that result in a prolonged reversible duration of action causing downregulation of pituitary GnRH receptors (after initial stimulation for the first 1-2 months of use), ultimately eliminating responsiveness to endogenous GnRH and, thus, decreasing LH and FSH secretion. Verification of adequate suppression can be confirmed by demonstration of prepubertal LH levels as measured in one of the aforementioned ultrasensitive assays measured just before a dose of therapeutic GnRH agonist or 1 hour after its administration in which the therapeutic agent is simultaneously used as an acute gonadotropin stimulator. Efficacy of treatment should be based on assessment of progression of Tanner stage and growth every3 to 6 months, and by periodic bone age and biochemical documentation of axis suppression. In the United States, the most commonly used GnRH agonists are monthly or 3-monthy intramuscular depot injections of leuprolide acetate and the histrelin implant.25,26 Histrelin is a 12-month subdermal implant requiring surgical insertion and removal on an annual basis either under local or short-term general anesthesia. Three-year efficacy studies with the implant have been performed showing sustained suppression of the HPG axis. Other available, but rarely used, FDA-approved GnRH agonist formulations are nafarelin acetate that is given twice daily by the intranasal route and leuprolide acetate that is given by daily subcutaneous injection. In children treated with depot leuprolide acetate, verification of adequate suppression of the hypothalamic-pituitary gonadal axis can be confirmed by demonstration of prepubertal LH levels using one of the aforementioned ultrasensitive assays measured 1 to 3 hour(s) after its administration in which the therapeutic agent is simultaneously used as an acute gonadotropin stimulator. Suppression in patients treated with histrelin may require acute GnRH agonist stimulation as random LH levels are not suppressed in approximately 60% of cases, with other criteria suggesting suppression. Regardless of treatment choice, ongoing documentation of axis suppression should be performed every 3 to 6 months. In girls, compared to serum levels of LH, FSH, and estradiol, those of free α-subunit (the shared component of heterodimeric glycoprotein hormones such as LH and FSH) respond more rapidly to implant removal and may represent the most sensitive indicator of gonadotropin recovery after cessation of histrelin treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree