Introduction
Each year more than 90,000 women are diagnosed with a gynecologic (ovarian, vulvar, vaginal, cervical, uterine, fallopian tube, or primary peritoneal) cancer in the United States. Radiotherapy is often used with curative intent with or without concurrent chemotherapy for locally advanced vulvar, vaginal, or cervical cancer. Women with high-risk features after surgery for cervical, uterine, or vulvar cancer may also receive adjuvant radiotherapy. The standard of care for women with locally advanced cervical cancer is external beam radiotherapy (EBRT) and intracavitary brachytherapy in conjunction with concurrent chemotherapy to increase local control and overall survival. For women with high-risk stage III/IV endometrial cancer, adjuvant pelvic or extended-field radiotherapy may be recommended, usually combined with systemic chemotherapy delivered either in a sandwich or sequential manner with or without concurrent radiosensitizing chemotherapy.
The target volume in women requiring pelvic radiotherapy includes the pelvic lymph nodes (obturator, external and internal iliac, and common iliac) as well as the presacral nodes in women with cervical cancer or those with endometrial cancer and cervical involvement. The primary target in women after hysterectomy includes the operative bed, which consists of the upper vagina and paravaginal tissues. For women with cervical cancer, the primary target would include the gross tumor volume, uterus, entire cervix, and parametrial tissues. The target would extend and cover the entire mesorectum with parametrial involvement. For women with involved or suspicious paraaortic lymph nodes, the field would extend to cover these nodes up to the level of the renal hilum.
EBRT was initially delivered with two or four fields to the pelvis or to the pelvis/paraaortic region, oftentimes with concurrent chemotherapy, for gynecologic cancers. Such a treatment combination can result in acute hematologic, gastrointestinal, and genitourinary toxic side effects as well as late morbidity. Recent randomized studies of intensity-modulated radiotherapy (IMRT) compared with three-dimensional conformal radiation therapy (3DCRT) for both uterine and cervical cancer after hysterectomy have demonstrated that IMRT can reduce the incidence of acute bowel toxicities. Importantly, acute hematologic toxicity in the setting of concurrent chemotherapy can result in dose reductions or delays in chemotherapy, which may result in compromised outcomes. Hematologic and gastrointestinal toxicities often limit the ability to deliver combined-modality therapy, as was observed when Duenas-Gonzalez et al. attempted to add weekly gemcitabine to weekly cisplatin concurrent with radiotherapy for locally advanced cervical cancer. Proton therapy for gynecologic malignancies has the potential to widen the therapeutic window and allow the testing of treatment intensification with novel therapies; it may also allow for potentially more conformal dose delivery and the possibility of dose escalation.
After hysterectomy
The radiotherapy technique for gynecologic malignancies has evolved in the last several decades. Initially, patients were treated with opposed fields or four-field conventional photon therapy, with fields defined by bony landmarks. With computed tomography (CT) imaging, the organs at risk (OARs) can be defined to reduce dose to nearby normal tissues. However, this still results in high doses of bowel irradiated. The recently completed NRG Oncology/Radiation Therapy Oncology Group TIME-C study (RTOG 1203) sought to test whether IMRT could reduce normal tissue toxicities in patients receiving posthysterectomy radiotherapy for either cervical or uterine cancer compared with 3DCRT. The primary end point was patient-reported bowel toxicity measured with the bowel domain on the EPIC (Expanded Prostate Cancer Index Composite) instrument. Results demonstrated that the EPIC bowel domain scores at 5 weeks of therapy for women receiving IMRT were higher (meaning less toxicity) than for women receiving 3DCRT. Given that smaller volumes of bowel receive radiation with protons, particularly in the low-dose region, it seems reasonable to test the hypothesis that proton therapy may further reduce bowel toxicity.
Lin et al. published the first clinical report on the use of pencil beam scanning (PBS) proton therapy for women who have undergone hysterectomy for either cervical or uterine cancer. In their series, 11 patients received PBS proton therapy using uniform scanning. They were treated using opposed lateral, two lateral and posterior, or two posterior oblique fields. Improved sparing in the low-dose region was noted for organs such as the small bowel, pelvic bone marrow, and bladder. Weekly or biweekly CT simulation scans were also obtained to confirm robustness relative to setup uncertainties.
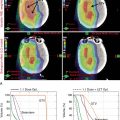
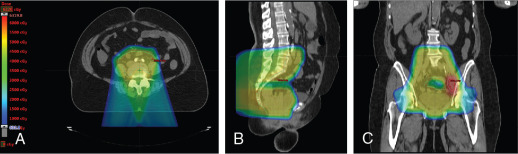
Xu et al. reported on seven patients with endometrial cancer who were treated with PBS to an extended field to include the pelvis and paraaortic region. They demonstrated that dosimetrically, PBS resulted in lower volumes of exposed pelvic bone marrow, small bowel, and bladder. No significant (grade 3 or higher) toxicities were observed in this population. All patients were treated with two posterior oblique fields. These early studies demonstrate the feasibility of treating patients with proton therapy. Other prospective studies are ongoing. Fig. 11.1 shows a patient who received PBS proton therapy after radical hysterectomy for a FIGO (Fédération Internationale de Gynécologie et d’Obstétrique) stage IIB cervical carcinoma. She also had a pelvic kidney that necessitated proton therapy to minimize the dose to that kidney.
Intact cervical cancer
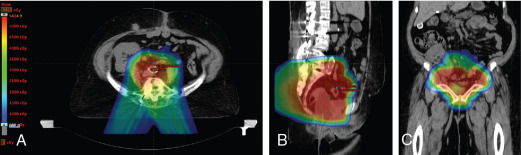
The use of brachytherapy is the standard of care in women with locally advanced cervical cancer receiving definitive chemoradiotherapy. Omission of brachytherapy has been associated with decreased survival. However, some rare situations may preclude the use of brachytherapy, such as a patient with bicornuate uterus (although interstitial brachytherapy may be an option), disease that obstructs the cervical os, or a patient who cannot tolerate sedation for the procedure. Arimoto et al. published the results of a series of 15 patients with gynecologic malignancies treated with high-energy proton beam radiotherapy in lieu of brachytherapy at Tsukuba University in the mid-1980s. Results at 2 years demonstrated local control rates and overall survival rates of 92.3% and 93.3%, respectively. All tumors were controlled if the dose was greater than 78 Gy. Two patients experienced transient radiation proctitis at 7 and 9 months after radiation. Clivio et al. also evaluated the use of intensity-modulated proton therapy (IMPT) in lieu of brachytherapy for locally advanced cervical cancer in a conceptual feasibility study. IMPT was planned with 5 fractions of 6 Gy to the cervix, including the macroscopic tumor as defined on magnetic resonance imaging (MRI) with a 5-mm margin. The doses to OARs, including the rectal wall, sigmoid wall, and bladder wall, were acceptable and within the dose constraints recommended by the GEC-ESTRO (Groupe Européen de Curiethérapie the European SocieTy for Radiotherapy and Oncology). , Given the toxicities associated with combined-modality therapy, it is reasonable to investigate radiotherapy techniques that would potentially reduce dose to OARs. Fig. 11.2 shows a representative example of a patient who was treated with PBS proton therapy for a FIGO stage IIIB cervical carcinoma. Posterior oblique beams were chosen with no exit radiation dose to the anterior abdominal organs. A multinational phase III study of concurrent cisplatin and gemcitabine with radiotherapy followed by consolidative cisplatin and gemcitabine for two cycles resulted in significantly higher toxicities and hospitalizations than the weekly cisplatin and radiotherapy, the standard of care. If we are to identify incremental improvements in combined-modality therapies for locally advanced cervical cancer, methods to reduce radiotherapy toxicities will be necessary.
Reirradiation
For patients who develop recurrent disease or a second primary after prior full-dose radiation to the pelvis, options may include surgical resection versus primary radiotherapy versus systemic therapy. Surgical resection may be possible depending on the clinical situation and has been used for other malignancies, including lung, esophageal, rectal, and breast cancer. , A patient with a central recurrence of cervical cancer treated with primary concurrent chemoradiotherapy may be a candidate for an exenterative procedure. However, a patient with a sidewall recurrence would not be a suitable candidate for surgery, but may be a candidate for reirradiation, depending on the extent of local disease as well as the status of disease elsewhere. Chemotherapy alone for gross disease is rarely curative on its own. The decision to deliver radiotherapy in an area of prior radiotherapy should consider multiple factors, including (1) length of time since prior treatment, (2) performance status, (3) options for alternative therapies, and (4) burden of disease. During the decision-making process, the potential for morbidity and increased late complications that this process could entail. Depending on the timing of the in-field recurrence, the disease could be considered a recurrence versus a new primary, and the ability to render curative therapy may also depend on the time course of the disease recurrence. Eifel et al. found that the hazard ratio for relapse peaked at the first year of follow-up and then fell off. They observed better survival rates among women who relapsed 3 years after initial therapy. Li et al., in a case report, described the treatment of a patient with a history of FIGO stage IIB cervical squamous cell carcinoma treated in the 1980s with conventional radiotherapy and low-dose-rate brachytherapy alone who subsequently developed a new vaginal squamous cell carcinoma in 2011. She was treated with limited opposed lateral passive scattering fields to treat just the primary fluorodeoxyglucose (FDG)-avid disease on positron emission tomography (PET)/CT imaging. She also received brachytherapy and now, more than 6 years after therapy is without evidence of disease; she did experience grade 3 radiation proctitis.
Simulation, target delineation, and treatment planning
A noncontrast CT simulation scan should be performed for dose calculation purposes, as contrast can shift the Hounsfield units (HUs) and compromise proton range calculations. If helpful, a contrast CT scan can be obtained subsequently and fused to the initial treatment planning scan for delineation of nodal volumes. Oral contrast may be given as well on subsequent imaging to aid in the definition of the large and small bowels.
Options for immobilization include a knee and foot lock indexed device that limits hip rotation and encourages flexion at the knees and hips when the patient is to be treated in the supine position. Arm positioning should be such that it does not interfere with beam paths and is reproducible on a daily basis. Use of a ring on the superior chest for a patient to hold is one option, as is use of a wing board with arms overhead. Although a prone position can result in a greater displacement of bowel, it may not be tolerable for patients. Such positioning also may not be reproducible on a daily basis without optimal immobilization. Devices such as alpha cradles or vacuum lock bags are not routinely used for proton therapy setups, as they may increase the proton range uncertainty and thereby compromise the precision of the beam.
To minimize rectal and bowel gas, the recommendation to the patient is to consume a low-fiber or low-residue diet before simulation and during therapy if possible. Prophylactic use of antigas tablets such as simethicone can also help promote regular emptying and reduced gas. For simulation and subsequent treatments, an endorectal balloon can also be used (filled with 50–100 mL of water).
Given the significant inter- and intrafraction variability in bladder filling, simulation with both a full and empty bladder is appropriate, with daily treatment given with a full bladder. This can be achieved by asking the patient to void fully and then drink 16 to 24 ounces (470–700 mL) of water approximately 45 minutes before their simulation and scheduled treatment time.
Target delineation
In patients requiring posthysterectomy pelvic radiotherapy, fiducials may be placed at the vaginal apex before simulation to better identify the vaginal apex for target delineation. Any artifacts caused by the fiducials or any high-Z materials, such as surgical clips, should be overridden for treatment planning purposes.
Depending on the clinical situation, MRI and PET/CT imaging may be useful for identification of gross disease, extent of invasion, and local recurrence. If these techniques are to be used for treatment planning for proton therapy, the scans should be performed while the patient is in the treatment position, ideally at the time of CT simulation with the identification immobilization device and fused with the noncontrast CT scan.
Clinical target volume and OAR contouring for a patient receiving proton therapy in the posthysterectomy setting is identical to that for a patient receiving IMRT. We recommend using the RTOG guidelines with the inclusion of the obturator nodal region, which should stop inferiorly when the obturator vessels enter the obturator canal. The nodal clinical target volume (CTV) should include the pelvic lymph nodes (external, internal, and common iliac), obturator, and presacral nodes when treating cervical cancer and endometrial cancer when there is cervical or parametrial involvement. Several groups have demonstrated significant vaginal motion depending on bladder/rectal filling. We therefore recommend using both full and empty bladder scans to delineate a vaginal internal target volume (ITV). Significant differences in rectal filling are possible as well, and we have treated patients with an endorectal balloon in place, both at the time of simulation and daily during treatment, filled with the same volume. Taku et al. have also demonstrated that with a daily endorectal balloon, the range of vaginal motion is smaller. Most patients tolerate this reasonably well. The other option is to simulate with and without an endorectal balloon and then treat daily without one, as is preferred at some institutions. A third option is to forego a balloon, but if the rectum is empty at the time of simulation, the anterior border of the ITV should be extended into the bladder by 2 cm, and if the rectum is full, the posterior border of the ITV should be extended into the rectum and within 1.5 cm of the posterior rectal wall. The proximal vaginal cuff should be included in the vaginal ITV.
The OARs to be contoured include small and large bowels, rectum, pelvic bone marrow (including bone marrow in the lower pelvis, ilium, and lumbosacral spine), kidneys, femoral heads, and bladder. The small and large bowels should be contoured within the field and 2 cm above the field.
Treatment of the paraaortics may be necessary for some women with either cervical or endometrial cancer, depending on the clinical situation. Treatment may be included as part of an extended-field treatment, or the clinical situation may be such that only the paraaortic region needs treatment. Typically, the fields are from the posterior direction and exit anteriorly into the bowel. This may not be a concern for patients who are receiving 45 to 50 Gy to treat subclinical disease. However, for patients with gross lymph nodes, the dose may be higher, upwards of 66 Gy. In those situations, the bowel relative to the CTV should be reviewed carefully on diagnostic imaging. If the small bowel, including the duodenum, is approximating the CTV, the neighboring bowel will receive the prescription dose, as the beam uncertainties at the end of the range are higher. In these situations, we may elect to treat with IMRT. We try to maintain the V55 of the duodenum to less than 15 cm .
A margin of 7 to 8 mm should be added to the nodal CTV to generate a planning target volume. This margin would account for variations in patient positioning and daily setup. The margin on the vaginal or primary ITV may be institution-specific, as it depends on how the ITV is contoured, but can range from 7 to 15 mm.
As discussed elsewhere throughout this book, proton beam ranges have uncertainties. To limit the effects of this uncertainty, robust optimization can be used, or a PBS-specific optimization structure can be created for planning purposes when a single-field optimization planning technique is used. Typically, this structure is created by adding to the CTV a margin of 3.5% of the beam range in the beam direction.
Treatment planning
Several-specific treatment-planning issues can affect proton range uncertainty and robustness that must be considered. Imaging artifacts must be minimized, as they can influence proton range calculations. For instance, high-HU markers placed on the skin surface to help mark the isocenter can influence range calculations, particularly because they will not be on the skin during daily treatment. Any surface markers therefore must be contoured and overridden with the HU for air (−1000). Other artifacts in tissue caused by other high-density materials (i.e., calcifications, surgical clips, vaginal fiducials) should be contoured and overridden with the average HU of the tissue that is immediately adjacent.
Bowel gas within the treatment field should be contoured and overridden with the average HU of neighboring tissue. Bowel gas will increase the proton range if it persists during treatment; however, the rationale for contouring the gas is that it will provide robust target overage in the event that the gas is replaced with tissue. In clinical scenarios involving significant bowel gas, using the HU override technique is suboptimal, and beam angles should be chosen to avoid the areas of high gas.
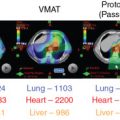
Weight changes can occur during course of therapy and particularly in patients requiring pelvic or abdominal treatment; this can change their anterior or lateral wall contours. For patients receiving pelvic radiotherapy for gynecologic malignancies, the preference for beam selection is posterior or posterior oblique, and therefore, this is less of a concern, but nonetheless it must be monitored. Berger et al. demonstrated that weight changes in patients receiving fractionated radiotherapy for cervical cancer did not result in significant changes to targets and OARs when a four-field (two laterals and two posterior obliques) IMPT plan was used, demonstrating the feasibility of IMPT.
As for beam angle selection, anterior fields are typically avoided to minimize the anatomical uncertainties in the beam path. Although lateral fields may be appropriate for pelvic treatments, they are not feasible for most paraaortic fields. Also, large patients with shifting abdominal tissue are not ideal candidates for lateral beams because the amount of soft tissue in the beam path could change on a daily basis. When lateral fields are matched to a superior posterior field, a smeared match line can be used to avoid match line changes. The match between these fields can be created by dose painting shallow gradients from each field at the match line using multiple optimization volumes.
Treatment delivery
If available, daily cone-beam or CT-on-rails imaging is appropriate. Weekly or biweekly CT imaging to evaluate potential changes in anatomy may also be sufficient if cone-beam CT imaging is unavailable. Daily kilovoltage imaging would be appropriate as well, with matching to bony anatomy or fiducials as clinically appropriate. Kilovoltage imaging may be sufficient to identify an endorectal balloon if used.
Conclusions
Proton therapy may benefit women with gynecologic malignancies by widening the therapeutic window. Future studies are needed to clarify the group of women who would benefit from proton therapy and to measure the benefits relative to other radiotherapy techniques.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree