Factor
Comments
Freeze rate
Rapid freeze > Slow Freeze
Cancer cells adapt to slow freeze
Temperature monitor
Strongly advises for monitor
Nadir temperature
Traditionally −40 °C was used
Recommend −20 °C
Thaw rate
Recommend passive thaw
Freeze cycles
Double freeze-thaw cycle advocated
Procedure
Patient Selection
Cryoablation is a treatment option that is suitable for patients with localized prostate cancer and negative metastatic evaluation. It can be offered to men who are unable or unwilling to undergo other forms of therapy. It can be safely used in men with prior pelvic radiation or significant bowel disease (i.e., Crohn’s disease) in which radical surgery is often complicated.
Men with negative metastatic evaluations, but high-risk features (PSA > 20, Gleason 8 or above) should be counseled about their risk of occult disease and offered lymph node dissection (AUA best practice) [15].
One of its major side effects is impotence and therefore it has been traditionally offered to men without potency or who are willing to lose potency.
Contraindications
Large prostate glands (>50 cm3 traditional)
Prior transurethral resection of prostate, specifically within 3–6 months
Cancers within the transition or central prostate zones
Procedure
Preparation includes a liquid diet the day prior to the procedure and a bowel preparation to cleanse the rectum. An enema is given the morning of the procedure.
Patients are given a first generation cephalosporin prior to the procedure. The procedure is performed under general or spinal anesthesia. They are placed in dorsal lithotomy position. The perineum and penis should be prepped completely with all hair removed from the perineum.
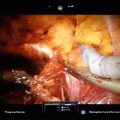
Most cryotherapy rectal probes are mobile and attach to the underside of the operative table. Once the patient is positioned, the rectal probe is inserted and the prostate is visualized. A brachytherapy grid is placed against the perineum. It is our practice to place two anchor needles once the grid is placed (Fig. 9.1).
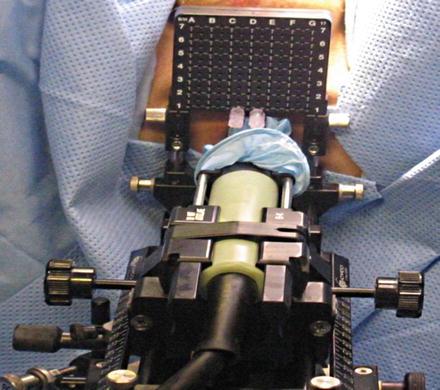

Fig. 9.1
Initial insertion of the transrectal ultrasound and grid
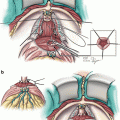
Probe placement sites are marked on the ultrasound screen. We ensure a distance of less than one centimeter between the probe sites and the prostate capsule, and a distance of less than 2 cm between probes. Seven probes are usually used. For small glands, five probes can be used. Two probes are placed at the apex of the prostate, two in the mid portion, and 3 at the base. The probes are placed under ultrasound guidance in both axial and sagittal views (Fig. 9.2). Sagittal view is used to ensure the bladder is not penetrated.
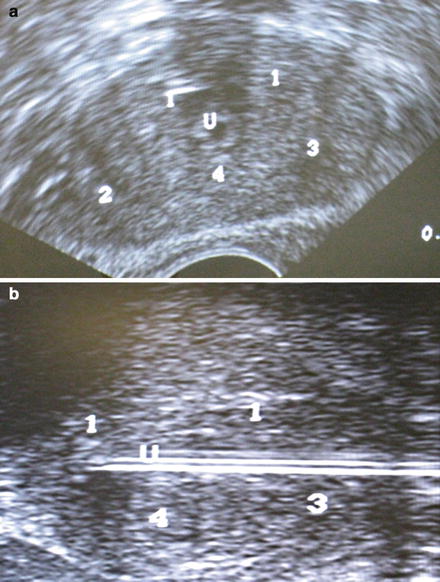

Fig. 9.2
(a) Transrectal ultrasound showing probe position. (b) Sagittal view showing expected probe locations. Note: Numbers correlate to freeze order, not probe number. White lines represent urethral catheter but probes are similar. U marks the urethra
Once all probes are in place, the temperature probes are then placed. We use five temperature probes that will allow monitoring of the freeze process and of vital structures. One probe is placed at each neurovascular bundle, one at the apex, one at the external urinary sphincter, and one at Denonvillier’s fascia.
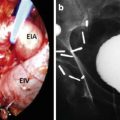
A flexible cystoscopy is then performed to ensure there has been no violation of the urethra or bladder neck by any of the probes. It is our preference to use a suprapubic catheter as it is more comfortable to patients and allows monitoring of post-void residuals. It is inserted at this point. A guide wire is inserted via the scope into the bladder. The guide wire is left in place while the scope is removed and is used to help the insertion of the urethral warming catheter. The bladder is kept partially inflated during the procedure.
Two freeze-thaw cycles are completed in line with current recommendations. The temperature is brought down to −40 and held for 2 min. The temperature probes are monitored, and the ice ball is monitored using ultrasound guidance in both the sagittal and axial view. The freeze cycle is adjusted accordingly. Once an adequate ice ball is formed which involves the whole prostate, the freeze cycle is stopped and all probes are thawed. The process is then repeated for the second cycle. Once complete, the probes are removed and the urethral warming catheter is left while in the recovery area. It is removed about an hour later, and the patient is admitted overnight (Fig. 9.3).
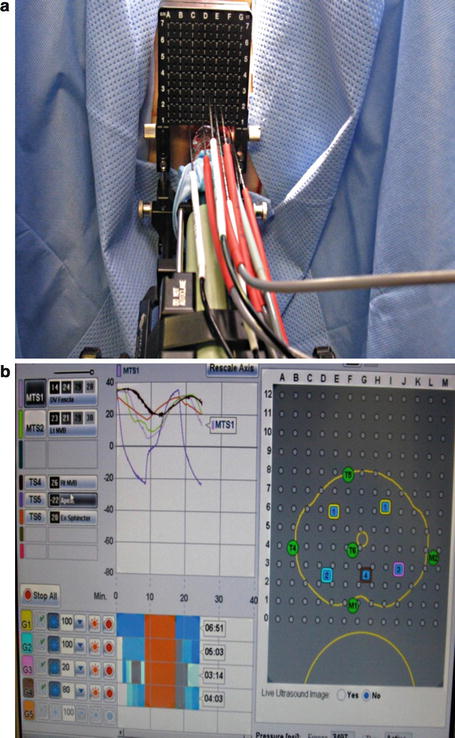

Fig. 9.3
(a) Probes inserted into perineum. Appearance after all probe are in place. (b) Appearance of the software. Temperature and freeze times are monitored with software
Most patients have perineal bruising. They are discharged with scrotal support and suprapubic drainage for 7–10 days. A fluoroquinolone is given for the duration of the catheter placement.
Outcomes
Defining Cyrotherapy Success
A persistent problem with whole gland cryoablation has been the difficulty in defining adequate treatment. Whereas radical prostatectomy and radiation have guidelines for failure, such as rising PSA nadir, the ASTRO criteria, or the phoenix criteria, this does not exist for cryotherapy. In the 2010 updated AUA Best Practice Statement [15], the conclusion was “As a consequence, meaningful comparisons of these reported outcomes from radical prostatectomy and radiation therapy to cryosurgery are not possible.” With this in mind, review of literature pertaining to whole gland ablation should be viewed with caution and should not be directly compared to other methods.
Filtering the available literature for mainly third generation cryogenic systems reveals a mixture of failure definitions. Most commonly used are the ASTRO and Phoenix criteria that were originally described for radiation (Table 9.2). Levy [17] examined the level of PSA nadir and its prediction of biochemical failure. Using the Cryo On-Line Registry (COLD), 2,427 patients were examined using PSA nadir cut-offs of less than 0.1 ng/mL, 0.1–0.5 ng/mL, 0.6–1.0 ng/mL, and 1.1–2.5 ng/mL. Increasing PSA nadir did show higher risk of treatment failure and the overall nadir was prognostic. Regardless of risk group, PSA nadir above 0.6 ng/mL was associated with significant 24-month biochemical failure. The authors caution that this cannot be used as a definition because the data was not correlated with disease-specific or metastatic-free survival.
Table 9.2
Definitions of commonly used criteria for biochemical failure
Criteria | Parameters |
|---|---|
ASTRO | Three consecutive rises in PSA |
Phoenix | PSA nadir + 2 ng/mL |
In this regard, one study deserves special mention as it is one of the only direct comparison done prospectively, non-inferiority, and randomized. Published in Cancer 2010, Donnelley [18] performed a randomized trial comparing external beam radiation and primary whole gland cryoablation. All study patients did receive neoadjuvant hormone ablation with LH-RH agonist. They excluded patients who had bulky T3 tumor, previous radiation, previous hormone treatment, or had undergone TURP within the past 3 months. Primary endpoint was 36-month failure post-randomization using the phoenix definition. At primary end point, there was a 17 % failure in the cryoablation arm and 13 % in the external beam radiation arm. There had been 10 deaths from prostate cancer, 5 in each arm during the study with a disease-specific survival of 96 % for both treatment groups. The conclusion was that cryoablation was not inferior to external beam radiation for the treatment of localized prostate cancer.
Primary Whole Gland Cryoablation
The gold standard in cryoablation is freezing of the entire prostate gland. In the early work, this method had significant morbidity including erectile dysfunction, urethrorectal fistula formation, and incontinence. As the treatment modality progressed, many of the features of our newest cryoablation systems have been added specifically to prevent or limit these complications.
Biochemical-Free Survival
Many studies have been published retrospectively reviewing cryoablation series with all three cryogenic generation systems (Table 9.3). A brief review will be placed here with emphasis on the third generation argon-based systems. For a full review of many of the articles published, please refer to Levy [19].
Table 9.3
Publications of primary whole gland cryoablation using third generation systems
Publication | Patients | Follow-up | Definition | Overall BDFS (%) |
|---|---|---|---|---|
Prepelica 2004 | 65 | 35 month | ASTRO | 83 |
Hubosky 2007 | 89 | 1 year | ASTRO | 94 |
Polascik 2007 | 50 | 18 month | PSA < 0.5 | 90 |
Jones 2008 | 1,198 | 5 year | ASTRO | 77 |
The 10-year data available has been generated from mixed systems with both nitrogen and argon in use as the freezing agent. Cohen [20] used nitrogen-based system and retrospectively analyzed 204 patients finding a 10-year biochemical disease-free survival (BDFS) of 56 % using the ASTRO criteria and 62 % using the Phoenix criteria. When they further divided in risk groups, the Phoenix definition was 80, 74, and 45 % for low, moderate and high, respectively. Statistically, these groups were different by long-rank test.
Shorter term follow-up has been published by several groups. Polascik [21] analyzed 50 men undergoing third generation ablation with an 18-month follow-up. They reported a 90 % BDFS using a threshold PSA of 0.5. With a median follow-up of 35 months, Prepelica [22] used an argon-based system with 83 % BDFS using ASTRO criteria. Finally, with 89 patients undergoing third generation cryoablation, Hubosky [23] showed 94 % at 1-year follow-up. To address the small amount of data available, the COLD registry was developed. In 2008, Jones [24] published a series of 1,198 patients with 5-year data from the registry. These patients when stratified by risk group and using the ASTRO criteria showed 84, 73, and 75 % BDFS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree