© Springer Science+Business Media New York 2015
Rakesh V. Khanna, Gennady Bratslavsky and Robert J. Stein (eds.)Surgical Techniques for Prostate Cancer10.1007/978-1-4939-1616-0_22. Transrectal Ultrasound-Guided Prostate Biopsy
(1)
Harvard Vanguard Medical Associates, An Affiliate of Atrius Health, 133 Brookline Avenue, Boston, MA 02215, USA
(2)
Glickman Urological and Kidney Institute, Cleveland Clinic Foundation, Cleveland, OH, USA
Abbreviations
ASAP
Atypical small acinar proliferation
AUA
American Urological Association
DRE
Digital rectal exam
ERSPC
European Randomized Study of Screening for Prostate Cancer
HGPIN
High-grade prostatic intraepithelial neoplasia
IPSS
International Prostate Symptom Score
PSA
Prostate-specific antigen
RCTs
Randomized control trials
SHIM
Sexual Health Inventory for Men
TRUS
Transrectal ultrasound
Introduction
Astraldi first described transrectal prostate biopsy in 1937 through a digitally guided approach [1]. A major innovation in the late 1980s was incorporating transrectal ultrasound (TRUS) with prostate biopsy, taking TRUS beyond static prostatic imaging. Hodge et al. demonstrated the use of TRUS to guide biopsies in a directed fashion using six evenly distributed biopsies across the parasagittal plane [2]. Instead of targeting palpable masses or hypoechoic lesions, the application of random biopsies has increased the detection of early stage prostate cancer and has contributed to the stage migration of prostate cancer present today. The topic continues to evolve today and this chapter looks to highlight the applications and techniques as well as controversies and innovations within the field of prostate biopsy.
Indications
TRUS-directed prostate needle biopsy is the gold standard for the diagnosis of prostate cancer. Traditional indications for prostate biopsy arise from prostate cancer screening and include abnormal digital rectal exam (DRE) or abnormal prostate-specific antigen (PSA). Prostate cancer screening has become a controversial topic [3] and is out of the scope of this chapter. The American Urological Association (AUA) 2013 Prostate Cancer Detection Guidelines states “There is no PSA level below which a man can be informed that prostate cancer does not exist.” Furthermore, “the Panel believes that the urologist should consider factors that lead to an increased PSA including prostate volume, age, and inflammation rather than using an absolute level to determine the need for a prostate biopsy”. Other indications include diagnosis of symptomatic prostatic cancer and diagnosis of failed primary therapy before initiation of second-line therapy. Indications for repeat biopsy include the men on active surveillance, diagnosis of multifocal high-grade prostatic intraepithelial neoplasia (HGPIN) [4], and atypical small acinar proliferation (ASAP) [5]. Contraindications to prostate biopsy include painful anorectal conditions, coagulopathy, immunosuppression, and acute prostatitis.
Preparation
Antibiotic Prophylaxis
The AUA Best Practice Policy on Urologic Surgery Antimicrobial Prophylaxis recommended prophylaxis in all men undergoing prostate biopsy with a fluoroquinolone or cephalosporin for duration no more than 24 h, citing level Ib evidence [6]. Further data supporting prophylaxis is provided in a meta-analysis of nine randomized controlled trials (RCTs), where bacteruria, bacteremia, fever, urinary tract infection, and hospitalization were all significantly reduced with prophylaxis. There are no definitive data to suggest that antibiotic for long-course is superior to short-course treatment, or that multiple-dose treatment is superior to single-dose treatment [7]. Due to rise in quinolone-resistant bacteria that is reported up to 30 % in men undergoing biopsy, practice now at the Cleveland Clinic is single-dose intramuscular aminoglycoside administration in addition to oral quinolone. In order to have 24 h serum levels, we now use levofloxacin 750 mg as the agent of choice. Alternatively, the introduction of rectal swab for target antimicrobial prophylaxis prior to prostate biopsy has been shown to be successful in reducing infectious complications and being cost-effective in very small studies [8]. This has not been adequately corroborated at the time of this writing and is complex to apply in clinical practice, so we have not adopted this to date.
Enema
Practice patterns in the community demonstrate that enemas are given routine pre-biopsy with rates up to 80 % [9]. While complications have not been shown to be reduced [7, 10], the hypothesis is that reducing the amount of feces in the rectum may allow for better imaging of the prostate by reducing the acoustic shadowing. However, the literature has failed to support the role of enemas, and our own publications have confirmed the urology literature overall that enemas do not provide any demonstrable benefit in the prostate biopsy setting. We do not recommend the use of enemas [11].
Positioning
Traditional patient positioning is the left lateral decubitus position with hip and knee flexion at 90°. Using an end-fire probe (described below) effectively mandates such traditional position to allow the hands to drop the probe handle in order to visualize the lateral aspects of the prostate. The lithotomy position can be used and is preferred in transperineal or template-guided biopsies, but may be associated with increased pain compared to lithotomy position [12].
Analgesia
The use of analgesia during prostate biopsy is varied with only 50 % of respondents reporting usage in 2004 [9]. Our observation is that most urologists have recognized the absolute value of analgesia and that most have adopted analgesic techniques since that time.
Three types of analgesia have been generally described and have been used in various combinations. The first, sedative analgesia, while effective is limited in application due to increased risk and cost of delivery. Intrarectal gel administration with 10 mL of 2 % lidocaine gel appeared to significantly decrease pain scores and offer analgesia prior to insertion of the ultrasound probe, although subsequent studies have failed to support its role [13]. Finally, periprostatic nerve block has been cited as the gold standard anesthesia for prostate biopsy [14].
A periprostatic block is achieved using 1–2 % lidocaine and injecting 2.5–5 mL with a long spinal needle. Meta-analysis of periprostatic nerve block demonstrated at least 35 % reduction in pain corresponding to a 1.6 point reduction in the visual analogue pain scale [15]. Various sites of infiltration have been described but most often cited is between the prostate base and seminal vesicle. Bupivicaine has been used with similar effect with the benefit of longer duration thus limiting rebound pain [16, 17]. While periprostatic block is preferred to intrarectal analgesia, combination of the two elements appears to achieve better pain control than each modality alone. This has been demonstrated only with prilocaine and not with intrarectal lidocaine [18, 19].
Most recently, the concept of pelvic plexus blockade with 1 % lidocaine to each side of the pelvic neurovascular plexus lateral to the tip of the seminal vesicle was shown to reduce pain during biopsy and pain perception after biopsy compared to traditional periprostatic blockade though further studies are necessary before this practice is standardized [20]. Furthermore, the procedure is significantly more complex to perform than is periprostatic block.
Ultrasonography of Prostate
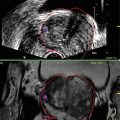
Though the role of TRUS in staging prostate cancer is poor, TRUS is the most common form of prostate imaging, largely to its role in directing biopsies. Endorectal probes typically transmit frequencies of 6–10 Hz. Higher frequencies allow for better image at reduced range; conversely, lower frequencies increase range with compromise of resolution. There are two types of probe geometry: end-fire and side-fire. End-fire probes require the handle to be angled away from the side of interest, using the anus as the fulcrum. Side-fire probes require twisting of the probe on a neutral axis for lateral visualization. Coupling medium of sonographic jelly or lubricant is helpful to create optimal sound transmission between the rectal mucosa and probe as well as the probe and the protective condom as ultrasound waves do not propagate effectively through air.
TRUS evaluation of the prostate includes scanning in both sagittal and transverse planes and calculating volume. Two settings commonly manipulated on the ultrasound machine are magnification and gain (brightness). Volume calculation is based in three dimensions, with anteroposterior (height) and transverse (width) dimensions measured in the axial plane at the widest diameter and longitudinal (length) dimension measured in the sagittal plane, using the ellipsoid formula.
Historically hypoechoic lesions have been associated with a prostate cancer and should be noted on TRUS. However, lack of hypoechoic lesion does not mean cancer is not present. Isoechoic areas demonstrated relatively equal per core cancer detection compared to hypoechoic areas (9.3 % vs. 10.4 % respectively) [21]. Hyperechoic lesions are less common than either hypoechoic or isoechoic lesion but display a similar propensity to cancer detection with a possible association with higher Gleason grade [22]. Based on unreliability of hypoechoic lesions to identify prostate cancer, most authors recommend including hypoechoic lesions in the systematic biopsy template, but not specifically targeting these lesions with additional cores.
Biopsy Techniques
The prostate biopsy is conducted through a spring-loaded, 18-gauge, needle core biopsy gun that is passed through the needle guide attached to the ultrasound probe. Most spring-loaded biopsy guns have a trajectory length of 2–3 cm. The previously noted skip area of 0.5 cm that results from advancement of the needle during fire has been disproven on our bench testing [23]. Our recommendation is that one should not intentionally pull back the needle 0.5 cm away from the tissue prior to firing unless the needle guide measurements indicate that the length from the rectal wall to the far side of the prostate is less than the needle’s trajectory.
Needle Path
The transrectal approach is most common in prostate biopsy and is the main focus of the chapter. Nevertheless, two other needle paths deserve mention, (1) transurethral and (2) transperineal. Historically, transurethral prostate biopsy was thought to better sample the transition zone but the current application is extremely limited [24]. Transperineal biopsy offers an approach to patients without a rectum, but also has gained favor in certain locations due to the potential to more readily biopsy the anterior prostate. With the rise of brachytherapy, the development of template-guided biopsies, and the avoidance of a transrectal approach due to multi-drug resistant E. coli, this approach may be seen more frequently. The diagnostic yield of transperineal biopsy versus transrectal biopsy has been varied with the only randomized, prospective trial to date demonstrating no differences of yield or complications between the groups [25]. Most transperineal biopsies are performed under general or regional anesthesia, which has limited adoption of this approach in many locales.
Probe Type
The type of ultrasonography probe appears to play a role in the detection of prostate cancer in transrectal biopsy. End-fire TRUS probe is superior in both initial and repeat prostate biopsy compared to side-fire probes [26, 27]. The ability of the end-fire probe to access high-yield areas such as the lateral zone, anterior tissue, and apex may explain the higher cancer detection rates. Nevertheless, armed with the knowledge that the anterior prostate should be sampled, it has been demonstrated that equivalent detection rates may be achieved with the side fire probe if technique is optimized [28].
Number of Cores
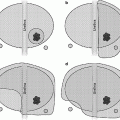
Prostate biopsy was initially guided by digital-directed sampling of palpable nodules or ultrasound identification of suspicious lesions. The cancer detection rate was then enhanced with the description of a sextant biopsy scheme that sample one core bilaterally from the base, mid, and apex [2]. Currently, the sextant scheme has been shown to be woefully inadequate for routine prostate biopsy for prostate cancer detection. The predominance of prostate cancer in the peripheral zone suggests under sampling of sextant biopsy and may explain false negative results. Extended biopsy protocols including 8–13 cores significantly enhanced the prostate cancer detection rate to upwards of 40 % compared sextant schemes (26 %) [29]. A large, multi-institutional study of community urologist involving 2,299 patients undergoing 12-core biopsy, overall cancer detection rate was 44 %, which is similar to the results of most academic single-center studies [30]. We recommend a 12–14 core extended-biopsy strategy for patient undergoing initial prostate biopsy, preferring to add two “extreme apical” cores based on our findings that these often identify unique cancers missed on the traditional 12 cores [31].
Saturation biopsies (defined today has minimum 20 transrectal cores taken at biopsy) were initially described in men with previous negative biopsies [32]. We have demonstrated that this is well tolerated under local anesthesia in the office setting thus reducing risk, time, and cost of using the operating room [33]. In our experience of over 1,000 saturation biopsies, we have not had increased complications compared to extended biopsy schemes [34]. By contrast, there is no role for transrectal saturation biopsy as initial prostate biopsy as cancer detection rates do not differ significantly from extended biopsy schemes even when stratified across PSA ranges [34].
Apical Biopsy
Apical biopsies are essential because of the high prevalence of cancers in this location, particularly anteriorly. In a true false negative biopsy, the anterior apical location is the most likely site of cancer missed [30]. The difficulty lies in accessibility especially for side-fire probes and patient pain during biopsy of the apex. We have reported that apical biopsy intolerance is not due to prostatic pain but is actually based on anal pain from sensory pain fibers below the dentate line of the rectum [35]. A rectal sensation test may avoid this pain by repositioning the needle about the dentate line, reaching an asensate area. Simply performing this maneuver may miss adequate apical biopsy by targeting the mid-gland. Aiming the probe handle craniodorsally pulls the rectal mucosa caudally, thus allowing painless apical biopsy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree