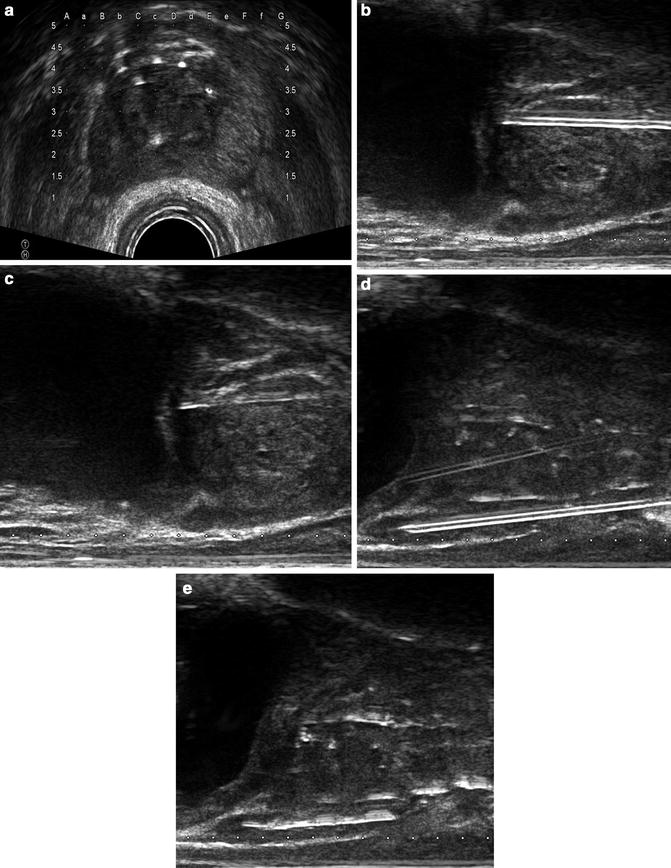
Fig. 8.1
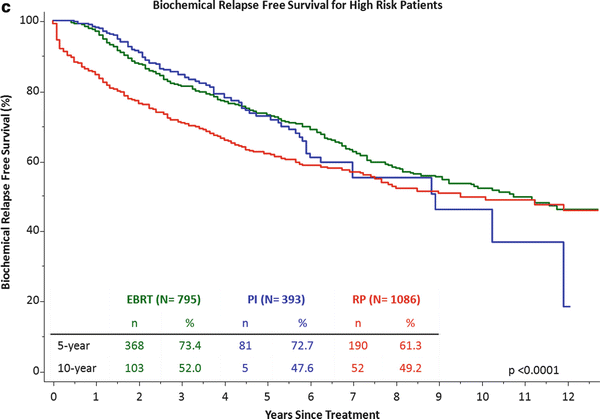
Cleveland Clinic institutional outcomes by NCCN risk category between 1996–2014. Part (a) is low-risk, part (b) intermediate-risk and part (c) high risk. The number at-risk at 5 and 10 years along with the biochemical relapse free survival is listed in each table. Radical prostatectomy (RP) is shown in read, permanent implant (PI) listed in blue and external beam radiotherapy (EBRT) listed in green
Personnel and Roles
To perform brachytherapy safely and efficiently a multidisciplinary team is necessary which may include the urologist, radiation oncologist, medical physicist, radiation therapist, anesthesiologist, and perhaps a medical dosimetrist. Furthermore, prostate brachytherapy has been shown to have a significant learning curve and therefore referral to a team experienced in prostate brachytherapy is recommended [25–27].
If brachytherapy is the recommended procedure, involvement of a medical physicist is necessary. Medical physicists are trained and certified in the planning, calibration, delivery, and quality control of radiotherapy. Their role is critical to the appropriate calculations required to deliver the radiation dose prescribed by the physician. A radiation therapist qualified in the handling and delivery of radiotherapy can aid in the logistical challenges inherent to radioactive sources as well as catheter loading during the procedure.
Anesthesia is recommended in the performance of brachytherapy although the type and delivery are institution specific. General anesthesia is most often used although some institutions prefer spinal anesthesia and obtain excellent outcomes. Local anesthesia with or without sedation is also possible although it requires an experienced physician and, while generally well tolerated, is occasionally more uncomfortable for the patient [28].
Radiation Biology and Physics
A basic understanding of radiation biology and physics can be useful when participating in the planning and the delivery of brachytherapy. Two broad categories of dose delivery can be identified: low dose rate (LDR) and high dose rate (HDR). LDR implants typically deliver dose at a rate of 0.01–2 Gy per hour and require weeks to months to reach full dose. HDR implants may deliver dose at a rate greater than 12 Gy per hour and require only minutes to deliver full dose to the target. LDR brachytherapy for prostate cancer is typically given via permanent seed implants using isotopes of 103Palladium, 125Iodine, or 131Cesium elements. HDR implants in the modern era typically employ 192Iridium via a remote afterloader. HDR brachytherapy as monotherapy is considered investigational by many investigators [29]. Table 8.1 lists various elements used in prostate brachytherapy for comparison.
Table 8.1
Physical properties of various elements used in prostate brachytherapy
Element | Avg. photon energy (keV) | Method of decay | Half-life (days) | Initial Estimated dose rate (cGy/h) |
|---|---|---|---|---|
103Palladium | 21 | EC | 17 | 21.2 |
125Iodine | 28 | EC | 59 | 7.0 |
131Cesium | 29 | EC | 9.7 | 34.2 |
192Iridium | 398 | β− (95 %) and EC | 73.8 | >1,200 |
198Gold | 412 | β− | 2.7 | 107 |
Measurement of Dose
Various methods for quantification of ionizing radiation dose are available and a general familiarity is necessary for the clinician comparing various techniques in radiotherapy, particularly between EBRT and prostate brachytherapy. The most clinically relevant measure of radiation dose today is the Gray (Gy) which is the SI unit of absorbed dose and defined as 1 Joule (J) of energy deposited per kilogram of tissue (J/kg). The rad, previously the standard, is equivalent to 0.01 Gy, or 1 cGy. Gray is a measure of energy deposited in tissue and has various biological effects dependent on a myriad of other factors. The sievert (Sv) is a unit defined as the human biologic equivalent or effective dose and is most relevant in radiation safety applications. For photons, 1 Gy is approximately equal to 1 Sv. Protons, having an increased mass and an increased relative biologic effectiveness, deliver approximately 2 Sv per 1 Gy absorbed. The rem (Röntgen equivalent in man), a previous standard, is equivalent to 1 rad or 1 cGy. It is important to remember that the biologic effective dose (BED) is a complex comparison particularly when made between brachytherapy and EBRT. The clinical BED is most related to the fraction size and number of fractions delivered but is also related to the quality of radiation (energy, photon vs particles such as protons), dose rate, the type of tissue in question, the rate of cellular repair, oxygenation, and the cell-cycle state of the tumor. The complexity of these comparisons explains the challenges encountered when attempting to identify the ideal radiotherapeutic approach to prostate cancer.
Dose Deposition: Energy and the Inverse Square Law
The energy of ionizing radiation is a key factor in determining the depth of tissue penetration. Higher energy photons travel further into tissue before attenuating as defined by the percent depth–dose (PDD) curve. Early kilovoltage units used for EBRT were ineffective in treating prostate cancer due to the inability to deposit dose deep into the pelvis. The key advantage of brachytherapy over EBRT is quantified by the “inverse square law” which states that the intensity of radiation emitted from a source is inversely proportional to the square of the distance from the source (8.1). This allows for relatively high doses to the tissue in contact with the source and a much lower dose to the normal tissue surrounding the target.
Inverse Square Law
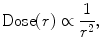
where r = distance from source.
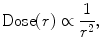
(8.1)
Treatment Planning and Dosimetry
Target Volume Delineation
Regardless of the technique used for delivering prostate brachytherapy, the target and the organs at risk (OARs) remain the same. The prostate, including the capsule and a margin surrounding the capsule, is the key target for localized disease. For intermediate and high-risk disease the proximal 1–2 cm of the seminal vesicles may be included in the target volume. Key OARs include the urethra, bladder, rectum, and penile bulb. It is not possible to deliver sufficient dose to the pelvic lymph nodes with transperineal prostate brachytherapy.
Contouring
“Contouring,” a common term in the field of radiation oncology, is the process by which targets and OARs are identified on imaging in order to calculate dose delivered to the structure. While automated algorithms exist, contours are always checked and edited by the physician. The urologist, radiation oncologist, and medical physicist vary in their degree of involvement in contouring based on institution and physician preference. The RTOG has assembled an expert panel to define an atlas of standardized references for contouring [30].
Generally the prostate should be contoured as the key target, sometimes coined the “gross tumor volume” (GTV) although it may be more appropriate to describe the prostate as the “clinical target volume” (CTV) as it is a volume concerning for disease which is not entirely composed of cancerous tissue. Some may describe a “planning target volume” (PTV) which, by strict definition, is an expansion on the CTV accounting for motion or set-up error. Given that brachytherapy is performed under real-time visualization of the target this standard definition of PTV is of debatable importance in the brachytherapy setting and is more applicable to EBRT. Many centers however do target an expansion of the prostate to account for risk of extracapsular extension which in surgical series appears to be within 4 mm in more than 90 % of cases [31, 32]. If an expansion is applied this may vary by NCCN risk group, but is typically approximately 5 mm at the apex and base, 2–3 mm anteriorly and laterally with no expansion on the posterior border. This could be considered part of the CTV, or otherwise PTV.
For ultrasound-based planning techniques commonly applied intraoperatively, images are sent from the ultrasound probe to the contouring software. The physician is then able to contour the edges of the prostate to define the CTV as well as the bladder or rectum as necessary. Any desired expansion can be easily performed at this time. After contouring, a plan can be generated which specifies a seed arrangement which best meets the targeted metrics (Table 8.2). For CT-based HDR plans or post-implant dosimetry verification scans the rectum is typically contoured from the rectosigmoid junction to the level of the anal verge. The peritoneal reflection and true rectosigmoid junction are difficult to delineate on imaging and therefore typically defined as the level where the colon begins to deviate laterally on axial imaging and begins to lose a circular shape. The bladder should be distended and the wall from the dome to the bladder neck should be included. The penile bulb, which is difficult to visualize on CT imaging without contrast, catheterization, or MRI fusion, begins inferiorly to the apex of the prostate and originates posterior to the urethra, having a circular shape in this region. Although debatable, some studies have related the dose delivered to the penile bulb to the risk of erectile dysfunction [42, 43]. The penile bulb contour should not extend to the pendulous portion of the penis. Small bowel and femoral heads are not typically of concern in brachytherapy and receive only background dose.
Table 8.2
Example of dosimetric goals for LDR and HDR brachytherapy
Monotherapy prescription | Prescription with EBRT | |
|---|---|---|
125I | 144–160 Gy | 110–125 Gy |
103Pd | 108–110 Gy | 90–100 Gy |
192Ir | ||
12–13.5 Gy × 2 fractions [39] | ||
Preplan | Post-plan | |
V100 | 100 % | 90 % Ideal, acceptable 80 % |
V150 | <50 % | <50 % |
V200 | <20 % | <20 % |
D90 | 115 % (110–130 %) | 100 % |
Urethra D max | <150 % (ideally <120 %) | <150 % |
Rectum D max | <1 cm3 receiving 100 % | <1 cm3 receiving 100 % |
Dose Prescription
After selecting and contouring the CTV and PTV, dose is prescribed to cover the intended target volume. For LDR monotherapy, common dose prescriptions include 144–160 Gy for 125I and 110–125 Gy for 103Pd. For a combined modality approach, the dose is typically 108–110 Gy for 125I, 90–100 Gy for 103Pd, and 10–22.5 Gy in 1–3 fractions for HDR (although no consensus in HDR dosing has been reached) [44]. Typical planning goals for 125I monotherapy include a preplanned D90 (minimum dose to 90 % of the PTV) between 110 and 130 % of the prescribed dose to reach a post-plan goal of 100 %. The preplanned V100 (volume receiving 100 % of the prescribed dose) should approach 100 % (at least >99 %) to obtain a post-plan V100 of at least 80 %, though ideally greater than 90 %. Regarding OARs, the maximum urethral dose should be less than 150 % of the prescribed dose (our institution target is less than 120 %) and the volume of the rectum receiving the prescription dose should be less than one cubic centimeter [45–47]. These dosimetric goals are summarized in Table 8.2. Figure 8.2 shows an example of a TRUS-guided preplan and the resulting post-implant evaluation on CT.
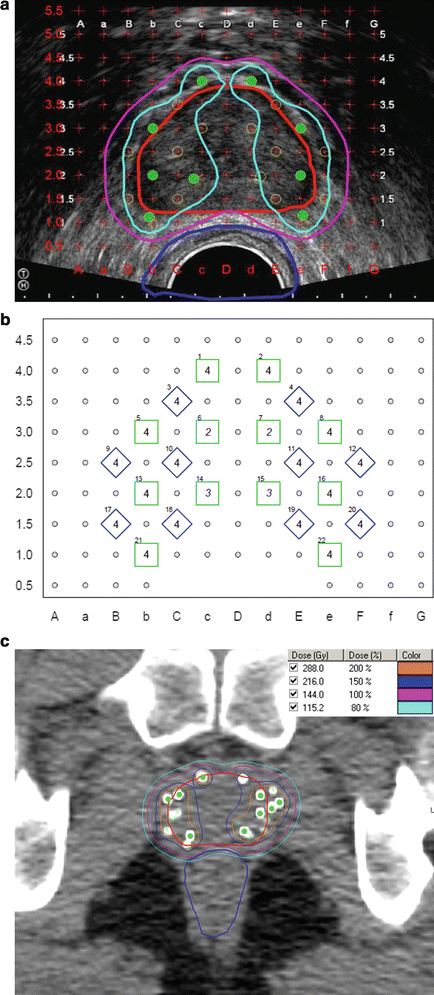
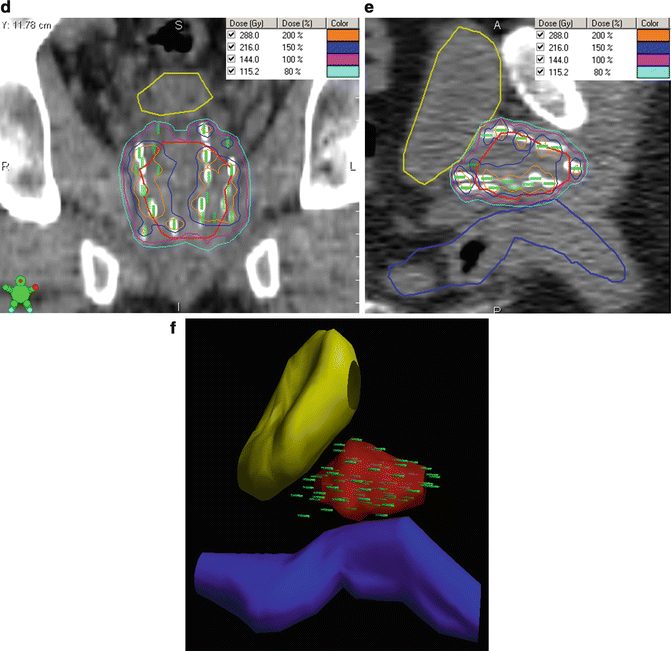


Fig. 8.2
TRUS-guided preplan and the resulting post-plan evaluation by CT. Isodose lines represent varying levels of radiation deposition. (a) TRUS-guided preplan showing the prostate CTV (red) with the 100 % isodose line (purple) and 150 % isodose line (light blue) with seed locations superimposed on the template grid (green). Note the urethral and rectal sparing. (b) TRUS-guided template showing needle spacing. (c) Axial post-plan by CT. (d) Coronal post-plan by CT. (e) Sagittal post-plan by CT. (f) 3D reconstruction depicting seeds within the prostate in comparison to the contoured bladder and rectal volumes
Isotope Selection
As stated in Table 8.1, there are various properties inherent to each isotope. Selection between LDR isotopes is based primarily on institution preference and there is debate in the literature regarding any potential clinical benefit of palladium, iodine, or cesium [48]. Iodine may have a logistical and cost-saving benefit as the half-life is extended and excess seeds can be saved for future procedures. The low dose rate of iodine allows for very low radiation exposure to personnel performing the procedure but may lead to a longer duration of sequelae. The decreased half-life of palladium potentially reduces the duration of sequelae and allows the option of implantation prior to EBRT in the setting of combined therapy. A randomized trial comparing 125I to 103Pd found no difference in biochemical outcome or long-term toxicity while suggesting a greater peak but faster resolution of acute effects with 103Pd as one might expect from the dose rate [49]. Theories of a low alpha/beta ratio for prostate cancer suggest that cesium may have a tumor control benefit due to increased rate of dose deposition although no clear clinical data support this [50]. Iridium is the element of choice for HDR brachytherapy.
Planning: Intraoperative vs. Preoperative
Planning of radiation dosimetry can be accomplished before, during, or after the implant procedure is performed. If the planning is accomplished days to weeks before the procedure, this is termed preplanning and has the logistical benefit of estimating the seed count although organ motion may be an issue. Intraoperative planning can be performed before the implant (intraoperative preplanning) or simultaneous with the implant (interactive intraoperative planning). With HDR brachytherapy treatment planning is performed postoperatively via CT performed with the implant in place and radiation is delivered via remote afterloader. Advantages to the preoperative technique include logistical flexibility and decreased time under anesthesia. The intraoperative preplanning technique, preferred by our institution, allows for accurate planning of the sources without significant interference of organ motion or deformation and improves dosimetric parameters compared to a preplanning technique [51]. If performed efficiently the increase in time under anesthesia is minimal. Post-planning with HDR brachytherapy has the potential advantage of optimization of source dwell times, allowing for some adjustment of the dose distribution after the implant. Despite this, implant (needle or catheter) position is critical and cannot be completely overcome by source optimization alone. If preoperative or intraoperative planning is performed, a postoperative verification scan is necessary.
Dosimetry and Planning: LDR vs. HDR Brachytherapy
HDR brachytherapy varies slightly in dosimetry and technique from LDR brachytherapy. During LDR brachytherapy permanent seeds are implanted whereas during HDR brachytherapy temporary catheters are placed (via the same TRUS-guidance technique) before the radioactive source is administered via remote afterloader. This technique eliminates the potential for seed migration or embolization and eliminates dose to treating staff. Another advantage is the ability to confirm the quality of the implant after the catheters have been placed but prior to radiation delivery. HDR can potentially deliver a more homogeneous dose profile due to post-planning and a higher energy of 192Ir is compared to the LDR isotopes (Table 8.2) [52]. A third potential radiobiologic advantage includes the hypofractionated schedule which may have a disease-control benefit based on the theories of a low alpha/beta ratio as mentioned above. Despite these advantages, careful technique is still required to ensure a high-quality implant. One potential disadvantage is the increased volume of normal tissue receiving low doses of radiation. The major disadvantage, however, is that most current schedules call for multiple implantation procedures (or for the implant to remain in place for an extended period of time over multiple fractions) as the use of single-fraction HDR monotherapy or boost remains investigational [53]. Ultimately, while there are multiple potential advantages to HDR brachytherapy, long-term randomized trials with patient-reported outcomes are yet to be conducted; therefore, brachytherapy technique remains the physician and institution’s choice.
Implantation Technique
LDR Technique
Procedural techniques will vary according to institution and type of implant required. Here a typical LDR implant using an intraoperative preplanning technique is described.
Prior to the procedure patients are encouraged to undergo routine preparation including avoidance of aspirin and anticoagulants for 5 days and abstaining from eating the night before. A bowel preparation using a Fleet enema or comparable can be helpful and is routinely administered 1 h prior to the procedure in our practice. Perioperative antibiotics are routine and typically include a cephalosporin or fluoroquinolone as indicated.
In the operating room the patient is placed in the exaggerated dorsal lithotomy position. Routine clean-contaminated surgical preparation is indicated. A complete surgical drape is institution specific but in our opinion is unnecessary. Ultrasound jelly placed into the rectum following rectal irrigation can be helpful prior to ultrasound placement. The TRUS unit should be capable of providing axial as well as sagittal images and include a stabilization device with template guidance. A template is attached to the TRUS unit to allow for accurate catheter insertion. Urethral visualization is usually possible with ultrasound and it is unnecessary to use a Foley catheter in the majority of cases. The use of a Foley can obstruct the view of the anterior prostate. If the urethra is unable to be visualized, consider injection of a small amount of either lubricant jelly or air if clinically necessary.
The entire prostate from 1 cm proximal to the base to 1 cm distal to the apex is imaged with 5 mm axial slices and images are sent to the planning software. The length, width, and height of the prostate are measured and volume is calculated both by the ultrasound unit and by the planning software. Correlation with the length of the prostate on sagittal view and the number of axial slices should be ensured to avoid error (e.g., a 4 cm prostate length should yield approximately 8–9 slices of prostate, 12–13 total captured slices).
Following image acquisition, commercially available planning software is then used to contour the CTV and OARs as detailed above. After calculation of the seed distribution, a final plan will display the number of sources and needles necessary and the correlated insertion location on the grid attached to the TRUS unit. At this point, ensure that the preplanned isodose lines are appropriate and that the preplan dosimetric goals have been met. Linked seeds ensure accurate separation and have been shown to decrease the rate of migration and embolization [54, 55]. Linked seeds should be used in the periphery with loose seeds inserted centrally near the urethra to allow for spontaneous or cystoscopic removal of a single source should such a situation arise. Likewise, we routinely use loose seeds for the inferior medial perirectal seeds as a precaution. Brachytherapy plans should typically be symmetrical and follow a modified peripheral loading technique [56].
During implantation under axial guidance (Fig. 8.3a), insert the needle through the template and firmly into the perineum. Begin with the needle in the axial coordinate farthest from the TRUS probe as image distortion may increase if the seeds are first delivered near the probe. An increased speed of needle insertion will minimize deflection of the needle. The deflection of the needle is related to the direction of the bevel and can be used to adjust for error. Once the needle is within the target coordinate, switch to a sagittal view to guide the depth as necessary. This use of sagittal ultrasound imaging eliminates the need for fluoroscopy in guiding the depth of needle insertion. Next, visualize the bladder wall and insert the needle in to the prostate base (Fig. 8.3b). At this point you will feel the increased resistance of the prostate capsule and also visually see increased deflection of the prostate on ultrasound. Once depth of insertion has been confirmed, deploy the seeds by holding the stylet in place and removing the needle (Fig. 8.3c). Ensure that the needle is not inserted further during the process of deploying the seeds as the bladder wall may be implanted. When inserting the inferior, perirectal needles extreme caution must be used to avoid deploying a source in, or immediately adjacent to the rectum. While it is occasionally necessary to pass a needle through the rectum for some patients, this can typically be avoided by inserting the needle into a higher than intended grid location and then guiding the needle inferiorly during insertion to avoid the rectal wall (Fig. 8.3d, e). A careful understanding of how this effects dosimetry is important and should be considered during planning. During placement we routinely use this technique for patients with steep rectal angles.