Pro
Contra
In vivo sensitivity testing
Fear of patients to leave the tumour in place
Strong prognostic value of pCR
Discrepancy in grading for core biopsy and definite specimen: risk of overtreatment
Higher rate of breast-conserving surgery/higher rate of operability/improved cosmetic outcome
Discrepancy between clinical and pathological nodal status: risk of undertreatment
Therapy monitoring possible
Prognostic value of pCR not demonstrated for all subtypes and regimens
Psychologic effect of tumour shrinking
Lack of guidelines for progressive disease under neoadjuvant therapy
Post-neoadjuvant concepts in case of non-pCR
Many patients such as those with, for example, non-inflammatory HR-positive and HER2-negative, low proliferative index breast cancer have no indication for chemotherapy due to a low expected relative and absolute treatment benefit and thus are not candidates for neoadjuvant chemotherapy. In cases of high tumour burden, they may benefit from primary endocrine therapy in order to perform breast-conserving surgery. In contrast, high-risk HR-positive/HER2-negative patients with tumours showing a high proliferation rate, high tumour burden in the breast and/or axilla or further risk factors such as grade 3 or high-risk classification based on a multigene assay may benefit from cytotoxic therapy and are therefore also candidates for neoadjuvant chemotherapy. The use of chemotherapy in HER2-positive and triple-negative breast cancer is common clinical practice. A lack of expression of oestrogen and progesterone receptors with or without overexpression of HER2 in combination with high proliferative activity, high tumour grade, high expression of Ki-67 or high genomic grade index is the main predictors for response to neoadjuvant therapy [11, 12]. Other predictors with a lower impact are age, non-lobular tumour type or early clinical response [13].
The indications for primary systemic therapy (irrespective of whether the recommended therapy is combination chemotherapy, an antibody-containing regimen or endocrine therapy) are easily summarized:
Inflammatory breast cancer
Inoperable breast cancer
To facilitate breast conservation surgery
If the same systemic therapy would also be indicated in the adjuvant setting
If adjuvant chemotherapy is likely to be advised and complex surgery is planned which may otherwise delay systemic therapy
If adjuvant chemotherapy is likely to be advised and the results of gene testing are awaited which may affect subsequent treatment decisions
Women must be counselled about the success or failure rate of PST in facilitating BCS if this is the aim, and a pretreatment MRI should be done to ensure unifocality if this is the aim. During and after PST, imaging with MRI to assess response may be useful to monitor response and guide subsequent therapy. In some cases a complete pathological and imaging response may occur, and in women wishing breast conservation therapy, it is important that a marker clip is placed in the tumour to facilitate subsequent tumour localization.
Following completion of primary systemic chemotherapy, surgery should take place between 4 and 6 weeks later to allow leucocyte counts to recover.
38.2 The Prognostic Relevance of Pathological Complete Emission (pCR)
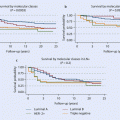
Numerous studies have shown that achieving a histopathological complete remission represents an independent prognostic parameter in the preoperative setting and therefore may serve as a surrogate marker for long-term survival of breast cancer [14, 15]. However, there is variability regarding both the probability and the prognostic validity of achieving a pCR among the distinct intrinsic breast cancer subtypes: while pCR is an important outcome parameter among patients with high-risk breast cancer subtypes (such as triple-negative breast cancer (TNBC) or HER-positive/hormone receptor (HR)-negative breast cancer), other breast cancer subtypes (such as low-risk hormone receptor positive (luminal A)) may have a favourable prognosis even in cases where there is residual tumour at the time of surgery. Nevertheless, achievement of a pCR is considered to be a relevant endpoint for regulatory authorities as the Food and Drug Administration in granting approval for new agents.
Another matter of debate is the optimal definition of pCR: at present there is debate about whether residual DCIS should be included in the definition of pCR [15, 16] and whether nodal response should be considered [17].
Finally, the simple dichotomy of chemotherapy response (i.e. pCR vs. any residual tumour) may not accurately reflect the heterogeneity of response. Consequently, optimized quantification of chemotherapy response has been suggested, for instance, by the use of semi-quantitative scoring systems such as the Residual Cancer Burden (RCB) [18] which combines histopathological tumour diameter, tumour cellularity, the number of axillary lymph node metastases and the diameter of axillary lymph node metastases by the use of a mathematical model to convert these into a single parameter that reflects the extent of chemotherapy response on a scale of 0–3. A value of 0 corresponds to a pCR. These values have been shown to correlate significantly with the prognosis of the patient in a semi-quantitative matter. Although the RCB score is used primarily in the USA, the use of this parameter can also be seen to be extending into Europe; however, this is largely in the context of clinical trials at present.
38.3 Regimens in Neoadjuvant Therapy
38.3.1 Neoadjuvant Endocrine Approaches
Although some physicians feel that primary endocrine therapy is mainly used in older postmenopausal women with comorbidities in order to achieve operability in large tumours or to avoid surgery at all in cases of severe risks for operative complications, basically all patients with endocrine responsive tumours and favourable risk factors such as ER/PR sensitivity, low nuclear grade or low Ki-67 [19] are candidates for a primary endocrine approach due to the low expected response rates to neoadjuvant chemotherapy [20]. In clinical practice this approach is rarely chosen for fit patients with good operability that are usually treated with endocrine therapy after surgery.
In general the endocrine regimen should be chosen according to the adjuvant data on endocrine therapy, but it is also helpful to look at the literature specifically looking at primary endocrine therapy.
In analogy to adjuvant studies, comparisons in the neoadjuvant setting have demonstrated the superiority of aromatase inhibitors over tamoxifen [21, 22]. Direct comparisons of letrozole, anastrozole and exemestane have shown similar efficacy for all three drugs [23].
The optimal duration of neoadjuvant endocrine therapy is unclear. Many patients are treated preoperatively for 4–6 months in clinical practice although 37% of patients may achieve maximal response only after 6–12 months [24].
For the reasons discussed above regarding which patients are candidates for neoadjuvant endocrine therapy, direct comparisons with neoadjuvant chemotherapy are rare. The few data indicating that in selected cases neoadjuvant endocrine therapy and neoadjuvant chemotherapy have the same response rates [25] reflect the fact that most patients in the chemotherapy arms should not have received neoadjuvant chemotherapy because of the favourable characteristics of their tumours: simply put, the biology of disease likely to respond to endocrine therapy is less likely to respond to chemotherapy and vice versa.
Ki-67 may represent a potential biomarker for use among patients undergoing primary endocrine therapy. In the IMPACT trial, Ki-67 measurements after 2 weeks of endocrine therapy were able to predict recurrence free survival [26]. The POETIC trial, which recruited 4000 patients until April 2014, was designed to test the significance of 2-week Ki-67 measurements during endocrine therapy as a prognostic marker for survival variables. However, results from this trial are not available yet [27]. Similarly, a 3-week approach regarding Ki-67 re-biopsy is investigated by the ADAPT trial. Pre- and postmenopausal patients with less than four positive lymph nodes are receiving 3 weeks of endocrine induction therapy according to guidelines. Risk of recurrence is furthermore assessed using the recurrence score: patients with a recurrence score of 11 or lower are treated with postoperative endocrine therapy alone; patients with a recurrence score of 26 or higher will receive neoadjuvant chemotherapy. In the group of patients considered to carry an intermediate risk of recurrence (i.e. recurrence scores 12–25), further therapy is decided based upon Ki-67 measurements following 3 weeks of endocrine therapy: patients with a Ki-67 higher than 10% after 3 weeks of endocrine induction therapy are classified as nonresponders and will receive neoadjuvant chemotherapy, whereas in cases of a drop of Ki-67 below 10%, patients are operated on and will stay on endocrine therapy according to guidelines [28]. This trial is also still recruiting patients.
Studies are evaluating combinations of endocrine therapy with agents targeting endocrine resistance such as PI3K inhibitors, AKT inhibitors and CDK4/CDK6 inhibitors in the neoadjuvant setting. Several trials investigating this approach are currently recruiting (◘ Table 38.2) [29].
Table 38.2
Clinical trials investigating neoadjuvant endocrine therapy combined with agents targeting endocrine resistance
Trial number and planned recruitment | Phase | Arms | Duration | Primary endpoints |
|---|---|---|---|---|
PI3K inhibitor | ||||
NCT02273973N = 330 | Phase II | Arm A: taselisib + letrozoleArm B: placebo + letrozole | 16 weeks | pCR |
NCT01923168N = 360 | Phase II | Arm A: BYL719 + letrozoleArm B: buparlisib + letrozoleArm C: placebo + letrozole | 24 weeks | pCR |
AKT inhibitor | ||||
NCT01776008N = 87 | Phase II | MK-2206 + anastrozole; goserelin acetate if premenopausal | Maximum four cycles of 28 days each | pCR based on Ki-67 values |
CDK4/6 inhibitor | ||||
NCT01723774N = 29 | Phase II | PD0332991 + anastrozole + goserelin acetate if premenopausal | Maximum four cycles of 28 days each | Complete cell cycle arrest (CCCA) based on Ki-67 values |
NCT0229801N = 306 | Phase II | Arm A: letrozoleArm B: letrozole (2 weeks) and then letrozole + palbociclib (12 weeks)Arm C: palbociclib (2 weeks) and then letrozole + palbociclib (12 weeks)Arm D: letrozole + palbociclib | 14 weeks | Change in Ki-67 values, cCR |
NCT02400567N = 132 | Phase II | Arm A: chemotherapy (FEC-Doc) Arm B: letrozole + palbociclib (12 weeks) | 18 weeks | Residual cancer burden |
38.3.2 General Considerations Regarding Primary Systemic Chemotherapy Regimens
Usually, neoadjuvant treatment regimens consist of combination chemotherapy regimens containing both taxanes and anthracyclines sequentially or simultaneously. However, anthracycline-free chemotherapy regimens may be considered a valuable alternative. It is suggested to use those chemotherapy regimens that would have been applied in the adjuvant setting rather than primary systemic setting. Response control during PST is important and is usually carried out by ultrasound evaluation every 6 weeks. However, data regarding an adjustment of the treatment regimen during the course of therapy in case of lack of sufficient response is insufficient despite the fact that there is data suggesting that patients with hormone receptor-positive breast cancer might benefit from switching to a non-cross-resistant regimen [30]. It is a common practice that patients not responding to PST and demonstrating tumour progression should stop treatment and immediately undergo adequate local therapy.
38.3.3 Choice of Therapy Regimens in HER2-Positive Breast Cancer
Choice of neoadjuvant therapy is largely decided upon based on established combinations of chemotherapy with trastuzumab or dual HER2 blockade. There is a large body of evidence suggesting that the poor prognosis associated with HER2 overexpression/amplification is counterbalanced by the high probability of benefit from HER2-directed therapies: in the neoadjuvant setting, the addition of the anti-HER2-directed antibody trastuzumab to chemotherapy is associated with a significant increase of pCR in several large-scale clinical trials leading to its approval as part of primary systemic therapy among patients with HER2-positive breast cancer (see ◘ Table 38.3).
Table 38.3
pCR rates of combined HER2-directed therapies in the neoadjuvant setting
NeoSphere (n = 417) | pCR (ypT0) | |
Trastuzumab + docetaxel | 29.0% | |
Pertuzumab + trastuzumab + docetaxel | 45.8% | |
Pertuzumab + trastuzumab | 16.8% | |
Pertuzumab + docetaxel | 24.0% | |
Neo-ALTTO (n = 455) | pCR (ypT0/is ypN0) | |
Trastuzumab → trastuzumab + paclitaxel | 29.5% | |
Lapatinib → lapatinib + paclitaxel | 24.7% | |
Trastuzumab/lapatinib → trastuzumab/lapatinib + paclitaxel | 51.3% | |
TRYPHaena (n = 225) | pCR (ypT0/is) | pCR (ypT0 and ypN0) |
FEC + pertuzumab + trastuzumab × 3 → pertuzumab + trastuzumab + docetaxel × 3 | 61.6% | 50.7% |
FEC × 3 → pertuzumab + trastuzumab + docetaxel × 3 | 57.3% | 45.3% |
Docetaxel/Carboplatin + trastuzumab + pertuzumab × 6 | 66.2% | 51.9% |
GeparQuinto (n = 620) | pCR (ypT0 and ypN0) | |
Epirubicin + cyclophosphamide + trastuzumab → docetaxel + trastuzumab | 30.3% | |
Epirubicin + cyclophosphamide + lapatinib → docetaxel + lapatinib | 22.7% | |
GeparSixto (n = 137) | pCR (ypT0 and ypN0) | |
Weekly paclitaxel + non-pegylated liposomal doxorubicin + trastuzumab + lapatinib × 18 | 36.8% | |
Weekly paclitaxel + non-pegylated liposomal doxorubicin + trastuzumab + lapatinib × 18 + carboplatin | 32.8% | |
GeparSepto (n = 1.200) | pCR (ypT0 and ypN0) | |
Weekly nab-paclitaxel × 12 → 4 × EC + trastuzumab + pertuzumab | 74.6% | |
Weekly paclitaxel × 12 → 4 × EC + trastuzumab + pertuzumab | 66.7% | |
Since the development of trastuzumab, several novel agents have been evaluated for the use among patients with HER2-positive breast cancer mainly in the primary systemic therapy setting. There is a large body of evidence suggesting that the small molecule lapatinib is inferior to trastuzumab with regard to rates of pCR as part of a combination regimen [31], but may increase pCR rates significantly if added to trastuzumab [32]. The increase in pCR, however, did not translate into a significant improvement of disease-free survival in a clinical trial using lapatinib and trastuzumab as part of an adjuvant treatment regimen (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation (ALTTO) Trial [33]). Therefore, lapatinib is not acknowledged as an optimal combination partner for trastuzumab in the potentially curative setting.
In contrast, data regarding the HER2 dimerization inhibitor pertuzumab seem to be more promising as findings from the neoadjuvant NeoSphere trial suggest [34]: Gianni and colleagues reported results obtained from 417 patients with HER2-positive breast cancer with a tumour size larger than 2 cm who were randomized to four 12-week treatment arms: trastuzumab/docetaxel, pertuzumab/trastuzumab/docetaxel, pertuzumab/trastuzumab and pertuzumab/docetaxel. The authors observed a significant increase in the rate of pCR by the addition of pertuzumab to trastuzumab and docetaxel (45.8% versus 29.0%). In the subgroup of patients with HER2-positive/HR-negative breast cancers, the pCR rate achieved by the use of dual HER2 blockade in combination with docetaxel was as high as 63.2%. Of note, among patients in the chemotherapy-free arm, dual HER2 blockade alone resulted in an impressive pCR rate of 16.8%.
Consequently, pertuzumab has received a label extension for the primary systemic setting in combination with trastuzumab and is registered in many countries as part of a standard neoadjuvant chemotherapy regimen. There is still, however, an ongoing debate as to whether this improvement in pCR will also translate into an improvement of prognosis. Survival analyses from the NeoSphere trials suggest a significant benefit regarding 3-year DFS (85 vs. 92%, HR 0.60 (95% CI 0.28–1.27)) [35]; however, data from the corresponding adjuvant Aphinity trials have not been reported yet.
38.3.4 Choice of Therapy Regimens in Patients with HER2-Negative Breast Cancer
Choice of chemotherapy regimen among patients with HER2-negative breast cancer is largely independent of hormone receptor expression status. Sequential or simultaneous combination chemotherapy containing both anthracyclines and taxanes has long been regarded as the standard primary systemic chemotherapy approach. Adjuvant studies have suggested benefit from the application of dose-dense chemotherapy if metastatic disease is diagnosed in more than three axillary lymph nodes [36] as well as in patients with TNBC [37].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree