Fig. 2.1
Projected increases in diabetes prevalence in different regions of the world, 2015–2040 (Source: International Diabetes Federation [1])
Risk Factors for Type 2 Diabetes
Both genetic and environmental factors underlie the development of T2DM [6, 7]. To date, more than 60 gene variants associated with T2DM have been identified; however, the effect size of these individual gene variants is rather modest [8–12]. The environmental risk factors strongly associated with T2DM risk include obesity, physical inactivity, history of gestational diabetes, hypertension, and dyslipidemia, among others (Table 2.1) [6, 7, 13]. These risk factors interact with genetic predisposition (indicated by a family history of diabetes and/or high-risk ethnic heritage) to promote the development of diabetes. Such a phenomenon was clearly demonstrated in the studies that showed a threefold increase in the rate of T2DM in recent Japanese immigrants to the United States compared with native Japanese [14]. Since a dramatic increase in disease prevalence over a relatively short time frame in humans is unlikely to be due to sudden new genetic mutations, environmental factors (notably changes in diet, physical activity, and perhaps microbial flora) probably trigger the surging diabetes rates among genetically predisposed populations. The exact mechanisms whereby these environmental triggers induce diabetes in genetically predisposed persons remain to be fully elucidated.
Table 2.1
Risk factors for type 2 diabetes
Physical inactivity |
Overweight/obesity |
First-degree relative with diabetes |
High-risk ethnicity |
Gestational diabetes or delivery of a baby weighing 9 lb or greater |
HDL cholesterol <35 mg/dl ± TG >250 mg/dl |
Hypertension (>140/90 mmHg or on therapy) |
A1C ≥5.7, IGT, or IFG on previous testing |
Conditions associated with insulin resistance: acanthosis nigricans, polycystic ovary disease, etc. |
History of cardiovascular disease |
Pathophysiology
Current understanding indicates that multiple pathophysiological defects underlie T2DM. Generally, at least eight unique pathophysiological defects are currently recognized in T2DM: insulin resistance, impaired insulin secretion, impaired glucagon suppression, increased lipolysis, exaggerated hepatic glucose production, incretin deficiency/resistance, maladaptive renal glucose reabsorption, and central nervous system defects (including impaired dopaminergic tone and dysregulation of satiety) [15–17] (Fig. 2.2). Insulin resistance can be inherited or acquired. Obesity, aging, physical inactivity, overeating, increased lipolysis, and accumulation of excessive amounts of nonesterified (free) fatty acids are known causes of insulin resistance. Normally, cytoplasmic long-chain fatty acids are transported into mitochondria as long-chain fatty acyl coenzyme A (LCFA-CoA) for beta-oxidation, a process that is gated by carnitine palmitoyl transferase (CPT)-1 and CPT-2 (the shuttle enzymes located in the outer and inner mitochondrial membrane). This shuttle process ensures that fatty acids do not accumulate excessively in the cytoplasm. Inhibition of that process leads to intracellular accumulation of long-chain fatty acids, which can induce lipotoxicity, cellular dysfunction, and cell death [16, 17]. Further, intracellular accumulation of long-chain fatty acids along with diacylglycerol (DAG) can activate certain isoforms of protein kinase C (PKC), leading to aberrant phosphorylation of the insulin receptor and consequent insulin resistance.
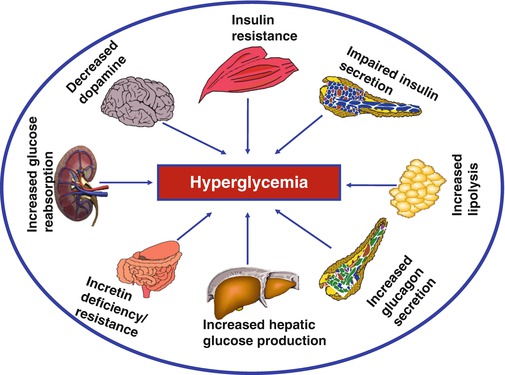

Fig. 2.2
The major pathophysiological defects that lead to the development and progression of type 2 diabetes. Many of the same defects (notably insulin resistance, impaired insulin secretion, lipolysis, and subnormal incretin response) at some degree of expression have also been described in people with prediabetes
Acetyl-CoA, a product of glycolysis in the Krebs cycle, can be converted to malonyl CoA by the enzyme acetyl-CoA carboxylase (ACC). Malonyl-CoA is the activated two-carbon donor required for fatty acid synthesis. Malonyl-CoA also is a potent inhibitor of CPT-1, thereby blocking the delivery and oxidation of fatty acids in the mitochondria. The result is accumulation of long-chain fatty acids in the cytosol and eventual lipotoxicity [17, 18]. Glucose abundance also increases the formation of intracellular DAG. Thus, multiple metabolic pathways link intracellular glucose abundance (usually derived from carbohydrate consumption) to impaired fat oxidation, fatty acid synthesis, accumulation of long-chain fatty acids, risk of lipotoxicity, and insulin resistance (Fig. 2.3). Among the potent interventions that have been demonstrated to ameliorate the pathological cellular and molecular processes leading to insulin resistance are caloric restriction (reduction of carbohydrate and fat intake), physical activity, and weight loss [19–27].
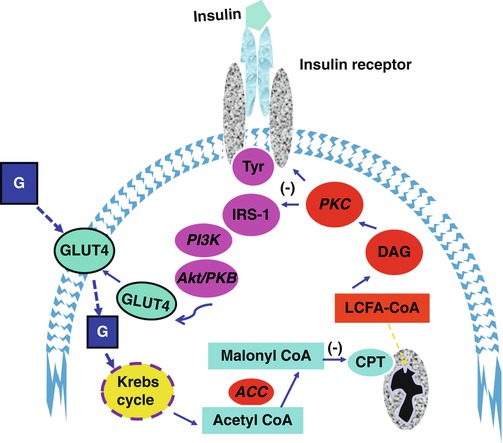

Fig. 2.3
Schematic diagram of insulin signaling pathways and interactions with glucose (G) and fatty acid metabolism. Increased intracellular glucose flux generates acetyl coenzyme A molecules which can be converted to malonyl coenzyme, a process catalyzed by acyl coenzyme A carboxylase (ACC). Malonyl coenzyme A inhibits carnitylpalmitoyltransferase (CPT), the mitochondrial enzyme that transports long-chain fatty acids (LCFA) into the inner mitochondrial space for oxidation. The resultant accumulation of LCFA in the cytosol is linked to insulin resistance via mechanisms involving protein kinase C (PKC) and altered phosphorylation of the insulin receptor
Prediabetes
The term “prediabetes” refers to impaired glucose tolerance (IGT) and impaired fasting glucose (IFG), two intermediate metabolic states between normal glucose tolerance and diabetes. IGT is defined by a plasma glucose level of 140 mg/dl to 199 mg/dl (7.8–11.1 mmol/l), 2 h following ingestion of a 75-g oral solution. IFG is defined by a fasting plasma glucose level of 100–125 mg/dl (5.6–6.9 mmol/l) [13] (Fig. 2.4). There is considerable overlap in the risk factors and pathophysiological defects that underlie T2DM and prediabetes. Although the exact sequence of evolution of individual pathophysiological defects has not been determined precisely, many of the defects coevolve during the pathogenesis of T2DM and are demonstrable even at the stage of prediabetes (Fig. 2.1) [28–38]. Estimates by the Centers for Disease Control and Prevention (CDC) in the United States indicated that there were ~29 million adults with diabetes and 86 million with prediabetes in 2014 [39]. Worldwide, there are more than 400 million people with prediabetes [1]. Individuals with prediabetes progress to T2DM at an annual rate of ~10 % [31, 32].
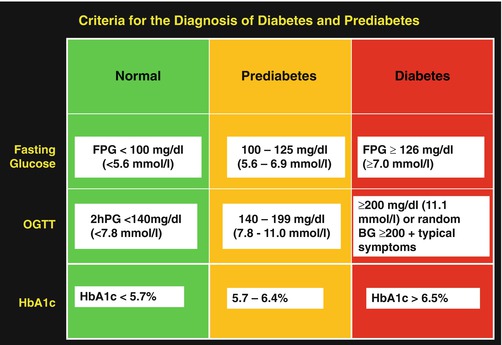

Fig. 2.4
Criteria for the definition of normal glucose regulation and diagnosis of prediabetes and diabetes. FPG fasting plasma glucose, 2hPG two-hour post-load plasma glucose, OGTT oral glucose tolerance test, using standard 75 g
Predictors of Progression from Prediabetes to Type 2 Diabetes
An analysis of six prospective studies [40] on progression from IGT to diabetes revealed the following features: (1) baseline fasting plasma glucose (FPG) and the 2-h OGTT glucose values are positively associated with diabetes risk; (2) the rate of progression from IGT to T2DM was exponential among subjects in the top quartile of baseline FPG but increased linearly with increasing 2-h OGTT glucose levels; (3) incident diabetes occurred at higher rates in Hispanic, Mexican-Americans, Pima, and Nauruan populations than among Caucasians; (4) the degree of obesity, as measured by the BMI, predicted T2DM risk in three studies with the lowest incidence rates of diabetes but not in the studies that recorded the highest incidence of T2DM; and (5) a family history of diabetes did not predict the risk of progression from IGT to diabetes in these studies, suggesting that genetic effects probably are fully established by the stage of IGT [40]. Thus, the magnitude of fasting and post-challenge dysglycemia (a reflection of insulin action and insulin secretion), ethnicity, and weight gain are major predictors of progression to T2DM.
Longitudinal studies in subjects from a high-risk population (Pima Indians) [41] with baseline normal glucose tolerance (NGT) indicated that weight gain, insulin resistance, and progressive loss of insulin secretory response to glucose predicted the development of T2DM [34]. Weight gain also predicted progression from NGT to IGT (5.2 kg vs. 2.6 kg in nonprogressors) and progression from IGT to T2DM during a 6-year follow-up period [34]. The greater weight gain in the progressors was accompanied by ~ 30 % worsening of insulin resistance and >50 % decline in acute insulin secretory response to intravenous glucose [34]. Weight gain also predicted incident T2DM in African-Americans in the Atherosclerosis Risk in Communities study [42]. It follows therefore that interventions that induce weight loss (e.g., diet, exercise, medications) could prevent progression from prediabetes to T2DM. In the prospective study of Pima Indians [34], progressive impairment of first-phase insulin secretion proved to be a critical determinant of progression from NGT to IGT and from IGT to T2DM. Progression from IGT to diabetes was associated with ~75 % decline in acute insulin secretory response to intravenous glucose [34]. A high concordance rate for impaired insulin secretion has also been reported among elderly identical twins discordant for T2DM (42), which suggests a genetic basis for pancreatic beta-cell dysfunction. The role of beta-cell dysfunction in predicting progression to T2DM indicates that interventions that prevent or replenish the progressive decline in insulin secretion can be expected to prevent the development of diabetes.
Predictors of Initial Transition to Prediabetes
In contrast to the numerous studies on the transition from prediabetes to T2DM [34, 40–42], information on the incidence of prediabetes among initially normoglycemic persons is scant. In a study of 254 Pima Indians with normoglycemia, 79 subjects (31 %) progressed to prediabetes (IGT) during a mean follow-up period of 4 years [43]. Based on those results, the incidence of prediabetes among Pima Indians can be estimated at ~8 %/year [43]. Of the 468 subjects with NGT at enrollment in the Baltimore Longitudinal Study on Aging (BLSA), over half were followed for at least 10 years [44]. By 10 years, 62 % of the initially NGT participants had progressed to prediabetes, yielding an incidence rate of prediabetes 6.2 % in the BLSA cohort (96 % of whom were European-American) [44]. Dagogo-Jack et al. followed 343 healthy African-American and European-American offspring of parents with T2DM in the Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) study and observed that 100 had developed incident prediabetes (IGT and/or IFG) during a mean follow-up period of ~3 years, without evidence of ethnic disparities [45]. Thus, among black and white subjects with parental history of T2DM, the incidence of prediabetes was ~10 %/year. These data indicate that the risk of incident prediabetes among offspring of parents with T2DM in the general United States population is similar to or higher than the risk observed among Pima Indians, a group with the world’s highest rate of T2DM [41]. The POP-ABC data underscore the importance of heredity, familial, and genetic T2DM. Taken together, these studies found that normoglycemic individuals develop prediabetes at an annual rate of 6–10 %, the higher rate being more likely among those with a strong family history of T2DM. Based on findings in the Pima Indian [34] and the POP-ABC [45] studies, the predictors of incident prediabetes include older age, male gender, overweight/obesity, lower insulin sensitivity, and impaired acute insulin secretory response to glucose [45]. Other predictors of incident prediabetes included food habits, physical inactivity, higher C-reactive protein, and lower adiponectin levels [45–47]. Obesity is a likely unifying factor that links the various pathophysiological mechanisms leading to dysglycemia. Comparison of several measures of adiposity indicates higher values in people who progress from normoglycemia to prediabetes compared with those who maintain normal glucose metabolism (Table 2.2).
Table 2.2
Selected baseline demographic and clinical characteristics of participants who developed prediabetes (progressors) compared to those who remained free of incident prediabetes during 5 years of follow-up in the POP-ABC study
Characteristic | Progressors | Nonprogressors | P-value |
|---|---|---|---|
Number | 111 | 232 | – |
White/black | 53/58 | 97/135 | 0.3 |
Female/male | 65/46 | 180/52 | 0.0003 |
Age (year) | 47 ± 8.9 | 43.9 ± 10.7 | 0.0017 |
Age 18–40/40–65 | 23/88 | 85/147 | 0.0030 |
Weight (kg) | 90 ± 20 | 83 ± 22 | 0.0036 |
BMI (kg/m2) | 31.4 ± 6.9 | 29.6 ± 7.4 | 0.0013 |
Waist (cm) | 99 ± 14 | 92 ± 16 | <0.0001 |
Female | 98 ± 12 | 91 ± 16 | 0.0006 |
Male | 101 ± 15 | 96 ± 15 | 0.14 |
Total fat mass (kg) | 32.0 ± 12.6 | 29.9 ± 14.0 | 0.0025 |
Female | 37.2 ± 12.0 | 32.1 ± 14.4 | 0.02 |
Male | 24.2 ± 9.1 | 22.4 ± 9.1 | 0.0004 |
Trunk fat mass (kg) | 16.6 ± 6.8 | 14.3 ± 7.3 | <0.0001 |
Female | 18.9 ± 6.7 | 15.1 ± 7.6 | 0.0064 |
Male | 13.1 ± 5.4 | 11.5 ± 5.5 | 0.07 |
Rationale for Primary Prevention of Type 2 Diabetes
There are compelling reasons why the primary prevention of T2DM ought to be an urgent policy priority in developing countries. Undoubtedly, the prohibitive costs of managing diabetes and its complications could easily overwhelm the budgets of many low- and middle-income countries. In many such countries, competing pressures from infectious diseases and periodic epidemics can easily relegate considerations for diabetes care to the bottom of the totem pole of noncommunicable diseases. However, the unique demographic (increased diabetes susceptibility at younger age and female preponderance) and the geographical (urban vs. rural) distribution of the diabetes provide clear targets for preventive intervention. Moreover, there is interplay between diabetes and chronic infectious diseases (e.g., tuberculosis) as well as between successful control of HIV infection and iatrogenic diabetes. But, the strongest argument for prioritizing diabetes prevention is the premium availability of effective tools that have been developed in landmark studies conducted in different parts of the world that established the feasibility of preventing T2DM [48–51].
Diabetes mellitus imposes a huge drain on national health budgets. The annual diabetes-related health-care costs exceed $240 billion in the United States [52]. Although precise figures are hard to come by, on average, developing countries spent at least 5 % of their total health expenditures on diabetes in 2010 [53]. The 5 % budgetary allocation is suboptimal and could be much higher if diabetes-related medical costs were to be adjusted to account for the >50 % rate of undiagnosed diabetes in developing countries [53, 54]. Optimal control of glycemia and related comorbidities is difficult and expensive to achieve in patients with established diabetes. Availability of antidiabetes medications, diabetes testing and monitoring supplies, laboratory support, and the professional care team (comprising of physician specialists, nurses, dietitians, diabetes educators, podiatrists, and other experts) is often elusive in many countries that face challenges in health-care infrastructure (Fig. 2.5). Where some or all of these essential resources for effective diabetes control are available, their distribution often is lopsided, and most rural areas are underserved. In the absence of comprehensive national health coverage, affordability of medications and self-management supplies, even when available, cannot be guaranteed for most low-income patients. For example, in sub-Saharan Africa, insulin security and affordability have been major concerns [55, 56]. Lacking cost defrayment by government or reimbursement by third-party insurers, the limitations imposed by the significant out-of-pocket costs of diabetes care translate to chronic suboptimal care, which increases the burden of diabetes-related complications [57].
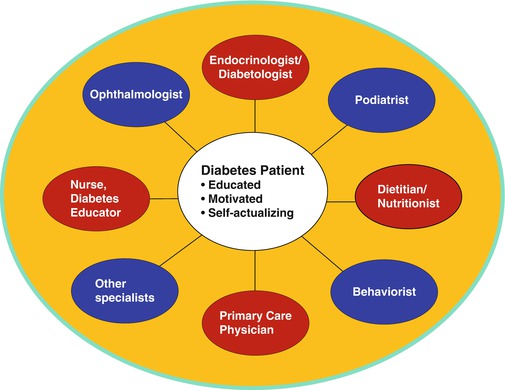

Fig. 2.5
Human capital investment in building a multidisciplinary team-based diabetes care service. A well-informed and highly motivated patient is critical to the success of diabetes care
Type 2 diabetes, a global epidemic, now ranks among the leading noncommunicable public health challenges of the present era [1, 4, 5]. The public health burden imposed by diabetes is underscored by the fact that diabetes now is the leading cause of blindness, end-stage renal failure, and nontraumatic limb amputations and a major contributor to heart disease, stroke, and peripheral vascular disease [57–60]. These complications can be prevented or delayed by achieving and maintaining excellent control of glycemia and comorbid conditions, such as hypertension and dyslipidemia [61–64]. However, the achievement of sustained glycemic control to the level necessary for prevention of complications often proves elusive, even in countries in the developed economies [65, 66] (Fig. 2.6). Optimal glycemic control requires a highly motivated patient, working with a diabetes care team comprising physicians and several cadres of clinicians (Fig. 2.5). The care processed involves the use of multiple medications; frequent clinic visits; adherence to challenging lifestyle prescriptions; performance of demanding self-management tasks; paying for cumulative costs of home blood glucose test strips; sustained engagement by a team of physicians, nurses, dietitians, diabetes educators, ophthalmologists, podiatrists, behaviorists, and other specialized professionals; and the implementation of sundry other recommendations [13, 67] (Fig. 2.5). Lacking the requisite resources and support for excellence in diabetes care, many patients in developing countries face the specter of chronic suboptimal control of glycemia and multiple-related risk factors. Consequently, the long-term complications of diabetes flourish unchecked. Ironically, many developing countries lack the infrastructure, technology, and human resources for adequate management of diabetes complications. Services like dialysis, renal transplantation, laser surgery for retinopathy, interventional cardiology, and rehabilitation services for amputees are not routinely available. It is, thus, self-evident that primary prevention of T2DM is an imperative for developing countries [68, 69].
Unique Vulnerabilities in Developing Countries
Data from surveys in developing countries indicate that diabetes predominantly affects younger age-groups: the majority of people with diabetes fall within the age range of 40–59 years, as compared to 60 years or older in developed countries [69]. It has also been predicted that future increases in diabetes numbers would affect all age-groups in developing countries, whereas in developed countries an increase is expected predominantly among persons older than 60 years, with a slight decrease in the younger age-groups [69]. This younger age predilection means that individuals in developing countries in their prime productivity years are the ones burdened with diabetes and its complications, with dire consequences on national economies. Besides the enormous direct medical costs, the additional lost productivity from absenteeism and presenteeism inflicts compounding negative effects on current and future economic performance in the developing world. Furthermore, the preponderance of diabetes in young women of childbearing age perpetuates a vicious cycle through the effects of intrauterine fetal programming for increased susceptibility to cardiometabolic disorders in postnatal life [70, 71]. A more recent vulnerability is the unexpected association of successful antiretroviral therapy in HIV patients with treatment-emergent metabolic perturbations, including diabetes, dyslipidemia, and lipodystrophy [72, 73].
Approach to Prevention of Type 2 Diabetes
Lifestyle Modification
Three landmark studies have demonstrated the efficacy of lifestyle intervention in preventing the development of T2DM in high-risk individuals [48–51]. All studies targeted persons with prediabetes (principally IGT and high-normal fasting plasma glucose). The lifestyle counseling focused on dietary intervention and increased physical activity and a weight loss target of approximately 5 to <10 % of initial body weight. The dietary intervention was aimed at encouraging participants to adopt healthy eating patterns and to decrease caloric consumption (by ~500–700 kcal/day) through selective reduction in saturated fat calories and limitation of excessive carbohydrate intake. The physical activity component motivated high-risk individuals to accrue 150–240 min of moderate-intensity activity per week (~30–60 min daily on 5 days or more each week). The target intensity (~55 % VO2 max) of physical activity is equivalent to walking at a brisk pace [48–51]. Compared with the control groups (who merely received passive health information), these lifestyle modifications proved remarkably efficacious in decreasing the rate of progression from prediabetes to T2DM during study periods that ranged from ~3 to 6 years [48–51].
Da Qing Study
Investigators in the Da Qing study (49) screened 110,660 men and women from 33 health-care clinics in the city of Da Qing, China, and enrolled 577 adults (mean age 45 years; mean BMI 26 kg/m2) with IGT. The participants were randomized by clinic to a control group or to one of three active treatment groups – diet only, exercise only, or diet plus exercise – and were followed every 2 weeks during the first 3 months and quarterly thereafter for 6 years.
The primary end point, development of T2DM at 6 years, occurred in 67.7 % of subjects in the control group, 43.8 % in the diet-only group, 41.1 % in the exercise-only group, and 46.0 % in the diet-plus-exercise group. Interestingly, relative decrease in diabetes incidence in the active treatment groups was similar in lean or overweight (BMI >25 kg/m2) subjects. After adjustments for baseline differences in BMI and fasting glucose, the diet, exercise, and diet-plus-exercise interventions produced 31 %, 46 %, and 42 % reductions in diabetes risk, compared with control [48]. However, there was apparently no additive efficacy of combined diet plus exercise versus either component of the lifestyle intervention.
Finnish Diabetes Prevention Study
In the Finnish Diabetes Prevention Study (FDPS) [49], 522 middle-aged subjects (mean age, 55 years; mean BMI 31 kg/m2) with IGT were randomly assigned to either an intervention or control group. Each participant in the intervention group received individualized sessions with a health counselor (approximately every 2 months) and was encouraged to aim for ~5 % weight loss through reduction of total and saturated fat intake and increased intake of fiber. The lifestyle participants also were instructed to increase their physical activity by ~210 min per week. The primary end point was development of T2DM (confirmed by OGTT). The cumulative incidence of diabetes after 4 years was 11 % in the intervention group and 23 % in the control group, a significant 58 % reduction in diabetes incidence. The mean weight loss in the lifestyle group was ~3.5 kg compared with ~0.8 kg in the control group. Improvement in insulin and preservation of insulin secretion also occurred differentially in the lifestyle group compared with control [49].
Diabetes Prevention Program
The Diabetes Prevention Program (DPP) enrolled 3,234 participants with IGT and high-normal fasting glucose and assigned them randomly to intensive lifestyle intervention (ILI), metformin, or placebo treatment [50]. The enrollees included representation from all ethnic and racial groups in the US population; persons of non-European ancestry constituted 45 % of the entire cohort. The ILI targets were a minimum of 7 % weight loss/weight maintenance and a minimum of 150 min of physical activity per week. Subjects in the ILI group received a 16-lesson curriculum covering diet, exercise, and behavior modification delivered by case managers on a one-to-one basis during the first 24 weeks after enrollment. Subsequently, monthly individual sessions and group sessions with the case managers were provided, to reinforce the behavioral changes.
After an average follow-up period of 2.8 years, the participants randomized to ILI showed a 58 % reduction in the incidence of diabetes, as compared with placebo [50]. This beneficial effect of lifestyle intervention was seen in all demographic subgroups defined by age, gender, race, or ethnicity. Furthermore, reversion to normal glucose tolerance (NGT) occurred in ~40 % of subjects in the lifestyle intervention arm, as compared with ~18 % in the control arm [50]. Participants who experienced reversion to NGT (even if transiently) were 50 % less likely to develop diabetes during long-term follow-up, as compared with those who had persistent IGT status [74].
Indian Diabetes Prevention Program
The Indian Diabetes Prevention Program (IDPP) randomized South Asians with IGT to four arms: control (with standard advice, N = 136), lifestyle modification (N = 133), low-dose metformin (N = 133), and lifestyle modification plus low-dose metformin (N = 136) [51]. Participants in lifestyle modification groups received counseling sessions aimed at promoting healthy eating habits (decreased intake of refined carbohydrates and fats and increased intake of dietary fiber) and boosting physical activity (at least 30 min daily). After a median follow-up of 30 months, the relative reductions in diabetes incidence were 28.5 % with lifestyle modification, 26.4 % with metformin, and 28.2 % with lifestyle modification and metformin [71]. Remarkably, these benefits of lifestyle modification occurred despite the lack of significant weight change in the two groups that focused on lifestyle change.
Translating Diabetes Prevention to Communities in Developing Countries
Collectively, the extant data show that lifestyle modification is remarkably and consistently effective in preventing the development of T2DM in high-risk populations in China, India, Europe, and the United States. To date, no large RCTs of diabetes prevention have been published from Africa, Latin America, Australia, New Zealand, the Middle East, or the Caribbean regions, although a number of pilot projects have been implemented or are ongoing. Some of these pilot projects have reported promising results among indigenous (Maori) New Zealanders [78]. There is overwhelming evidence that lifestyle modification is a more compelling approach to diabetes prevention than medications. However, the landmark lifestyle intervention programs that achieved remarkable success in preventing diabetes were designed as randomized controlled trials (RCTs) and conducted predominantly at academic medical centers. The protocols of many of the RCTs entailed frequent clinic visits, utilized specialized multidisciplinary teams (including physicians, nurses, dietitians, psychologists, exercise physiologists, and others), and consumed substantial resources and support from institutions and funding agencies. Notably, all protocol-mandated services and interventions were provided at no cost to participants in the diabetes prevention RCTs, many of whom also received incentives and stipends. Thus, the odds were stacked heavily toward success in these RCTs, and it is crucial to determine whether the sterling results obtained in the landmark RCTs could be reproduced in the community, without all of the inbuilt advantages, specialized professionals, and resources. Some community initiatives are currently underway to determine the feasibility of community programs for diabetes prevention [79, 80]. The United States Centers for Disease Control and Prevention (CDC) has been training and certifying community diabetes prevention personnel under the aegis of the National Diabetes Prevention Program [81]. Programs using trained lay persons to deliver adaptations of the DPP lifestyle intervention to groups (rather than one-on-one sessions) in the community have been shown to produce promising results [79, 80, 82].
Keys to Translation
In general, the keys to the translation of any new concept or discovery related to health consist of (1) adequate comprehension, evaluation, and acceptance of the new information within a specialized professional community; (2) processing and conversion of the new information into the professional knowledge base and dissemination to a wider circle of professionals and students; (3) diffusion of the conceptual information to the general public, through demonstrative education by convinced professionals; and (4) adoption of evidenced-based changes in practices and behaviors, triggered by the new knowledge. The preceding construct of translational cascade is somewhat akin to the sequential steps in Prochaska’s transtheoretical model of behavior modification: pre–contemplative, contemplative, preparation, action, and change [83, 84].
Dissemination of Diabetes Prevention Knowledge
The preponderance of evidence from randomized clinical trials on the efficacy of lifestyle intervention in preventing T2DM has established the rationale for diabetes prevention as self-evident among health professionals. However, the screening for prediabetes and institution of prompt lifestyle counseling have not become routine practice, even in economically advanced countries. Bridging the hiatus between philosophical acceptance of the idea of diabetes prevention among health professionals and the adoption of pragmatic steps to detect and act upon high-risk persons requires a methodical approach.
The information and knowledge base of health-care professional must be steered toward a more pragmatic approach to primary prevention of diabetes through a number of established approaches. First, curriculum development in primary and high schools should begin to introduce seminal data from the behavioral components of the landmark diabetes prevention studies within a wider context of instruction in wellness and health promotion. Second, students in schools of medicine, nursing, pharmacy, and allied medical fields should receive exposure to formal instruction in the design and key findings of the major diabetes prevention studies, again, within the wider context of wellness and health promotion. Third, mastery of the principles and methods of primary prevention of T2DM ought to be a priority item in the core curriculum of residency training programs for physicians. The latter can be implemented through lectures and workshops by intramural experts and invited speakers. Finally, practicing physicians in all disciplines (including internists, family physicians, endocrinologists, cardiologists, nephrologists, general and specialist surgeons), podiatrists, nurse practitioners, and other primary care providers must have their knowledge base updated to include the tenets of primary prevention of diabetes.
Operationally, the increased awareness about diabetes prevention at the primary care level should lead to increased zeal for screening and detection of individuals with prediabetes (often relatives of patients with established T2DM). Once individuals have been diagnosed with prediabetes (Fig. 2.4), appropriate referral for lifestyle counseling can follow, as recommended by the American Diabetes Association [13].
Convinced health-care workers and their families become conduits for the dissemination and diffusion of diabetes prevention ideas in targeted segments of the public (hospital communities, established patients and their relatives, schools, neighborhoods, social media and outlets, religious forums, etc.). Most human societies have encountered diabetes and are probably preconditioned for receptive attention to diabetes campaigns, having been primed by awareness of the more obvious complications, such as blindness, amputation, and end-stage kidney disease. Such a preconditioning of societal awareness of diabetes and its complications bodes well for the dissemination of ideas regarding the rationale and feasibility of diabetes prevention. Yet, the fact that T2DM can be prevented by modest caloric restriction and physical activity is yet to enter folklore. That gap in the popular consciousness (despite the raging global epidemic of diabetes) presents an enormous opportunity for leadership by health-care professionals and civic leaders. To convince and motivate large segments of society for preventive action against diabetes requires coordinated efforts at the local, regional, state, national, and international leadership levels. Creative programs anchored by ministries and departments of health, information, education, and other agencies would also be important catalysts for public education, awareness, and action. The expertise and contributions from philanthropic organizations and other nongovernmental bodies, especially local, national, and international diabetes associations, can be invaluable during the process of program building and facilitation.
Strategies for Diabetes Prevention in the Community
Five key elements can be distilled from the lifestyle intervention protocols utilized by the DPP and other major diabetes prevention trials. Most or all of these strategies can readily be adapted for widespread application in the community.
- 1.
Selection of persons at risk
All diabetes prevention trials targeted, screened, and enrolled a defined group of at-risk persons, using well-known risk factors for T2DM (Table 2.2). The merit of that approach is underscored by the finding that the participants randomized to placebo did in fact develop diabetes at an alarming rate (~12 % per year in DPP, ~18 % per year in IDPP) [50]. Thus, the published criteria used for selecting at-risk persons for diabetes prevention appear to be of high fidelity and can be adopted for translation of diabetes prevention in the general populace. Specifically, a positive family history of T2DM in first-degree relatives, overweight or obesity (using ethnic-specific BMI cutoffs), and a fasting plasma glucose in the range of 96–125 mg/dl predict a high yield of eligible individuals for community diabetes prevention efforts. The appropriate BMI cutoff for identifying overweight subjects appears to be >22 kg/m2 for Asians compared with >25 kg/m2 for most other ethnicities [85, 86]. The inclusive age range for the published diabetes prevention studies was >25 years for DPP and Da Qing studies, 40–65 years for FDPS, and 30–55 years for IDPP [48–51]. None of the published diabetes prevention studies enrolled individuals younger than age 25 years, which is a major limitation, given the increasing prevalence of T2DM in children and adolescents [87, 88]. Obesity and physical inactivity are major risk factors for T2DM in children, as in adults. Other risk factors include female gender, ethnicity, family history of T2DM, peripubertal age, and intrauterine exposure to diabetes [89–91]. Because the long-term complications of diabetes become established over a >10-year period, the epidemic of T2DM in children and adolescents predicts dire consequences for patients at the prime of their youth [92]. Primary prevention of childhood T2DM, therefore, is of utmost public health importance. For the aforementioned reasons, it is desirable for community diabetes prevention initiatives to lower the inclusive age for screening at-risk persons well into the childhood and adolescent years. A school-based pilot study sponsored by the US National Institutes of Health showed that obesity and diabetes risk factors can be decreased by lifestyle intervention in sixth grade pupils [93].
- 2.
Delivery of physical activity intervention
The physical activity component of lifestyle intervention in the reported diabetes prevention studies was of moderate intensity (~55 % VO2 max) and duration (~30 min daily in the DPP). Out of an abundance of caution, participants in the DPP lifestyle intervention arm underwent submaximal cardiac stress testing prior to commencement of the physical activity program [94]; however, that was not a routine requirement for physical activity in the majority of diabetes prevention trials [48, 49, 51]. It must be stressed that physical activity was well-tolerated in the DPP, and no untoward cardiovascular events or musculoskeletal injuries were reported. A routine requirement for prescreening with cardiac stress would be a serious logistical and economic hindrance to the widespread translation of diabetes prevention and may not be necessary for the majority of free-living individuals who are candidates for diabetes prevention in the community. The DPP exercise goal of 150 min per week was similar to that prescribed in the Malmo [95] and Da Qing [48] studies but lower than the 210 min per week prescribed in the Finnish study [49]. The DPP and all other landmark studies have demonstrated the efficacy, tolerability, and safety of moderate-intensity physical activity (150–210 min per week) as used for the prevention of T2DM [48–51, 95]. Walking was the preferred activity for the vast majority of participants in these studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree