Primary Cutaneous Lymphomas
Primary cutaneous lymphoma is defined by an accumulation of malignant lymphoid cells in the skin without evidence of extracutaneous disease at the time of diagnosis. The distinction between a primary cutaneous lymphoma and a nodal lymphoma with secondary cutaneous involvement is important and markedly impacts evaluation, staging, prognosis, and therapeutic management. The term primary cutaneous lymphoma encompasses a heterogeneous group of extranodal non-Hodgkin lymphomas, and in 2005 the World Health Organization (WHO) and European Organisation for Research and Treatment of Cancer (EORTC) endorsed a consensus classification system that defines three categories of primary cutaneous lymphomas: cutaneous T-cell and NK-cell lymphomas, cutaneous B-cell lymphomas, and precursor hematologic neoplasms/immature hematologic malignancies (Table 79.1).1 This chapter will review the primary cutaneous lymphomas in the context of this consensus system. A slightly modified classification system was released by the WHO in 2008.2
FIGURE 79.1. Pian fungoide, from Jean-Louis-Marc Alibert’s atlas of dermatoses, Descriptions des Maladies de la Peau.

 CUTANEOUS T-CELL AND NK-CELL LYMPHOMAS
CUTANEOUS T-CELL AND NK-CELL LYMPHOMAS
Cutaneous T-cell lymphoma (CTCL) is the most common primary cutaneous lymphoma. In the United States, 71% of the 3,884 cases of primary cutaneous lymphoma diagnosed during 2001–2005 were CTCL.3 Similarly, 78% of primary cutaneous lymphoma diagnoses recorded in the Dutch and Austrian Cutaneous Lymphoma Group registry over 1986–20021 and 85% of diagnoses in the Central Cutaneous Lymphoma Registry of the German Society of Dermatology over 1999–2004 were CTCL.4
CTCL subtypes include mycosis fungoides (MF); CD30+ lymphoproliferative disorders; extranodal NK-/T-cell lymphoma, nasal type; subcutaneous panniculitis-like T-cell lymphoma; adult T-cell leukemia/lymphoma; and primary cutaneous peripheral T-cell lymphomas. MF is the most common CTCL, responsible for 54% of CTCL diagnoses in the United States over 2001–2005.3 CD30+ T-cell lymphoproliferative disorders and cutaneous peripheral T-cell lymphomas represent the majority of the remaining cases of CTCL. The remaining CTCL subtypes are extremely rare and represent <1% of primary cutaneous lymphomas.
Mycosis Fungoides
MF is the archetype cutaneous lymphoma. The first case of MF was reported in 1806 by the French dermatologist Jean-Louis-Marc Alibert in his atlas of dermatoses, Descriptions des maladies de la peau (Fig. 79.1). After Alibert released his depiction of MF, approximately 300 similar cases were reported over the next decade, and in modern times approximately 1,500 new cases of MF were diagnosed during 2001–2005.3 MF is a disease of skin-homing CD4+ T-helper cells5 and is most commonly diagnosed in men (male:female ratio of 1.6–2.0:1) with a median age at diagnosis of 55 to 60.1 Significant advances in MF therapy have been made over the past 200 years, but the precise etiology responsible for the development of MF remains elusive.
Cutaneous Disease
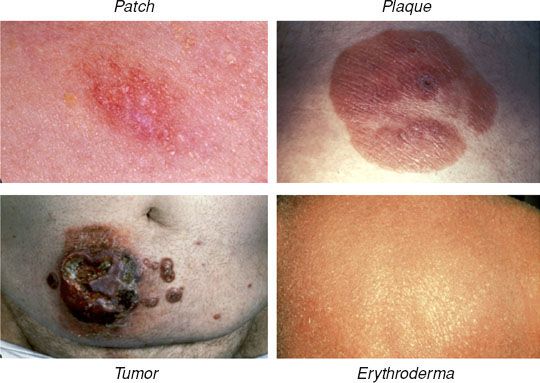
Pruritus, either diffuse or localized to areas of involved skin, is the most common symptom associated with MF. Ulcerated lesions may also cause patients significant pain. Cutaneous lesions in classic (or Alibert-Bazin) MF follow a predictable evolutionary course, and a consensus statement from the International Society for Cutaneous Lymphomas (ISCL), U.S. Cutaneous Lymphoma Consortium (USCLC), and EORTC defines the lesions found in distinct phases of MF.6 In the premycotic phase a small number of red, scaled, macular or patchlike lesions develop in sun-shielded areas of the skin such as the trunk, pelvis, and extremities. These early lesions are unstable and usually regress, followed by development of new lesions. Biopsies of premycotic lesions are rarely diagnostic due to a paucity of malignant lymphocytes in the lesion. With increased deposition of malignant T cells in the skin, the lesions become increasingly durable, and persistent cutaneous patches are characteristic of the patch phase. The ISCL/USCLC/EORTC consensus definition of an MF patch is “any size lesion without induration or significant elevation above the surrounding uninvolved skin: poikiloderma may be present”.6 As the lesions become more densely infiltrated by both malignant and reactive lymphocytes, they evolve into plaques with thickened and raised borders in the plaque phase of classic MF. Plaques are defined by the ISCL/USCLC/EORTC as “any size lesion that is elevated or indurated: crusting or poikiloderma may be present”.6 Plaques may evolve into cutaneous tumors, which the ISCL/USCLC/EORTC defines as “any solid or nodular lesion ≥1 cm in diameter with evidence of deep infiltration in the skin and/or vertical growth”.6 The presence of cutaneous tumors designates the tumor phase of MF. MF may also progress to or present with erythroderma, in which a diffuse erythema involves >80% of the skin surface area.6 Representative images of patches, plaques, tumors, and erythroderma are shown in Figure 79.2.
Leukemic CTCL and the Sézary Syndrome
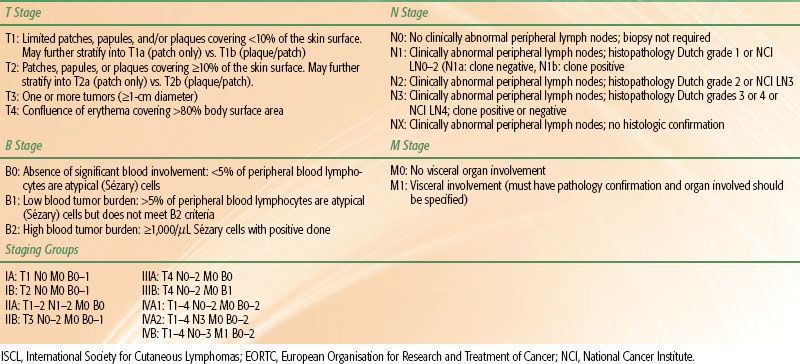
In rare CTCL cases circulating malignant T cells are identified in the peripheral blood. This phenomenon, sometimes referred to as leukemic CTCL or the Sézary syndrome (SS), most commonly occurs in association with erythroderma but may occur in patients with minimal cutaneous disease.1 The circulating malignant T cells (also called Sézary cells) are atypical T cells with hyperconvoluted nuclei seen at analysis of peripheral buffy coat smear. The circulating cells most commonly possess a CD4+/CD7– or CD4+/CD26– immunophenotype, and flow cytometry allows for quantification of the proportion of circulating malignant T cells. In addition, presence of a dominant circulating malignant clone may be demonstrated by evaluation of the T-cell receptor (TCR) by polymerase chain reaction (PCR) or Southern blotting. The degree of tumor burden in the peripheral blood is denoted as B0 (≤5% atypical or Sézary cells seen on examination of buffy coat smear), B1 (>5% of cells on buffy coat analysis are atypical, but further criteria meeting B2 disease are not met), or B2 (combination of presence of a dominant T-cell clone in the peripheral blood identified by PCR or Southern blot and either ≥1,000 Sézary cells/mm3, an increased amount of CD3+ or CD4+ T cells with CD4:CD8 ratios >10, or increased quantities of abnormal T cells defined as loss of CD7 in >40% of cells or CD26 in >30% of cells).6 If peripheral blood is evaluated for a circulating clone by PCR or Southern blot, B0 or B1 disease may be stratified into B0a or B1a (absence of circulating clone) or B0b or B1b (presence of circulating clone). The mechanism responsible for the development of leukemic disease in MF is unknown and in some cases may simply reflect disease progression. In other cases erythroderma and circulating disease develop simultaneously, which has historically been referred to as SS. The distinction between SS and erythrodermic MF with leukemic involvement (or SS syndrome preceded by MF) has been a point of controversy, and it remains unclear whether the two diseases are distinct or merely variants of one another. At present, SS is specifically defined as the combination of erythroderma with B2 disease.6
FIGURE 79.2. Representative images of cutaneous lesions in mycosis fungoides. (From Smith BD, Wilson LD. Management of mycosis fungoides. Part 1. Diagnosis, staging, and prognosis. Oncology 2003;17:1419–1428, with permission.)
Extracutaneous Disease
In advanced stages of disease MF may progress to involve regional or distant lymph nodes or extracutaneous organ systems (most commonly the lungs, oral cavity, pharynx, or central nervous system), and these sites may cause patients significant pain or functional impairment.7 Of note, in advanced cases of MF the extent of cutaneous or extracutaneous disease may vary significantly in patients of the same clinical stage. Clinical trials that report results by stage alone may therefore be difficult to interpret. The ISCL/USCLC/EORTC consensus statement on clinical end points and response criteria should facilitate improved communication of the degree of disease and response to treatment in future clinical trials.6
Molecular and Cellular Pathophysiology
Examination of early phase MF lesions by hematoxylin and eosin staining commonly reveals epidermotropism, a profound infiltration of lymphocytes into the epidermis. As the lesions progress and become thickened, epidermotropism is gradually lost as the lymphocytes begin to localize more diffusely in the skin. Only a fraction of the skin-homing lymphocytes in MF are malignant, and the malignant T cells may be identified by visualization of their small to medium-sized hyperconvoluted (or cerebriform) nuclei and surrounding lacunae, which give them the impression of being surrounded by a halo. A Pautrier’s microabscess (Langerhans cell surrounded by atypical T cells in the epidermis) is a less common but pathognomonic histologic finding associated with MF.1,5 The malignant cells in MF are CD4+ T-helper cells, which most commonly express a CD3+, CD4+, CD45RO+, CLA+, CCR4+ immunophenotype. Evaluation of the TCR profile of malignant T cells in MF reveals a predominant TCR rearrangement indicative of clonal dominance in a majority of cases.
The specific stimuli and molecular events responsible for activation and development of clonal dominance of a specific T cell are unknown. Genetic profiling studies have identified a number of chromosomal deletions and duplications associated with MF/SS, and microRNA profiling is beginning to reveal distinct molecular profiles associated with different phases of MF. In addition to the molecular aberrations intrinsic to the malignant T cells, the cutaneous microenvironment is also likely to play a key role in the development and maintenance of disease.5,8–11
Diagnosis and Evaluation
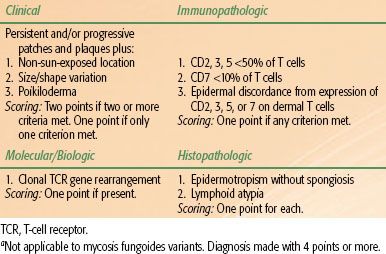
Evaluation of a patient with suspected or recently diagnosed MF should include a comprehensive history/physical examination, biopsy of cutaneous lesion(s) and suspicious lymph nodes, and appropriate laboratory and imaging studies. The ISCL has designed a diagnostic algorithm for early-stage MF (not applicable to MF variants) (Table 79.2),12 and specific recommendations for patient evaluation have been released by the ISCL/EORTC.13
History and Physical Examination. The duration of symptoms, evolutionary course of cutaneous disease, and presence or absence of B symptoms should be determined. The percentage of body surface area involved by disease and presence or absence of cutaneous tumors should be documented, and a thorough evaluation of the lymphatic system should be performed. Appropriate images of cutaneous lesions should be recorded so that disease progression or response to therapy may be monitored.
Skin Biopsy. Biopsies should be taken from a minimum of two distinct sites of disease. Lesions of the greatest induration will be most likely to yield a diagnosis due to greater numbers of malignant cells. However, scaled lesions are more likely to show epidermotropism. Tissue should be evaluated with hematoxylin and eosin staining, immunostaining for surface marker expression profiles (including CD2, CD3, CD4, CD5, CD7, CD8, CD20, CD30, CD26, CD56, TIA1, granzyme B, βF1), and PCR for clonal TCR rearrangement. Slides should be reviewed by a dermatopathologist.
Excisional biopsies of enlarged or otherwise suspicious lymph nodes (fixed or matted lymph nodes, or lymph nodes ≥1.5 cm or ≥1 cm in the head and neck) should be evaluated as described previously. When multiple suspicious lymph nodes are encountered, the choice of lymph node to be excised should be based on size, fluorodeoxyglucose (FDG) avidity, and location, with priority given to the largest lymph node draining an affected area of skin or the lymph node with the highest standardized uptake value (SUV) on positron emission tomography (PET) scan. If all other factors are equal, priority should be given first to cervical nodes, followed by axillary and then inguinal nodes.13
Laboratory and Imaging Studies. Laboratory studies should include a complete blood count, chemistry panel, liver function tests, and lactate dehydrogenase (LDH). Examination of the peripheral blood for circulating disease by PCR and flow cytometry should also be considered, particularly for patients with erythroderma, nodal, or extracutaneous disease. Bone marrow biopsy should be considered if peripheral blood or extracutaneous organs are found to harbor disease.13 Computed tomography (CT) of the chest, abdomen, and pelvis is recommended in the evaluation of all patients with MF with the exception of patients with patch/plaque disease limited to 10% or less of the body surface area. In these cases a chest x-ray or nodal ultrasound may suffice. PET scanning was shown to be more sensitive than CT in identifying involved lymph nodes, and the role of PET in MF continues to evolve.14
Staging and Prognosis
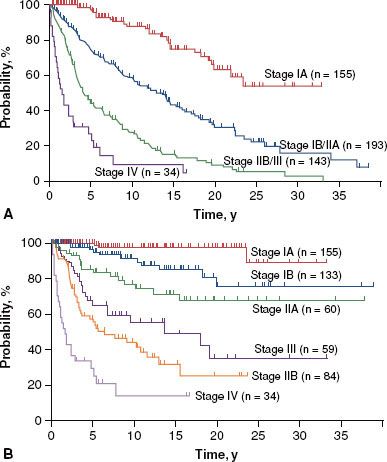
MF is presently staged using the modified TNMB system proposed by the ISCL/EORTC (Table 79.3).13 Based on a review of 525 patients with MF (staged using the previous 1979 TNMB system), the majority of patients present with early stage disease (30% IA, 25% IB, 11% IIA, 16% IIB, 3% IIIA, 8% IIIB, 6% IVA, and 1% IVB). Only 7% of patients presented with peripheral blood involvement. The correlation of clinical stage to overall and disease-specific survival is presented in Figure 79.3.7 Similar results were obtained in a validation study of the current staging system.15 Of note, stage IA MF is not associated with any increase in mortality risk in comparison to an age- and ethnicity-matched control population, and is associated with only a 16% rate of disease progression at 20 years of follow-up.7
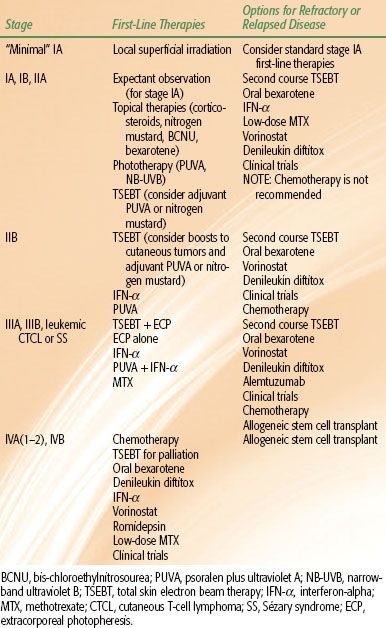
Skin-Directed Therapy
MF is not generally considered to be curable, although extended periods of disease-free survival after treatment have been reported. Efforts to treat MF are primarily focused on preventing progression and ameliorating symptoms. In early-stage disease a number of skin-directed treatments are effective and should be considered as first-line therapy. Topical therapies include corticosteroids (complete response [CR] rates of 63% for T1 and 25% for T2 disease),16 nitrogen mustard (CR rates of 76% to 80% for stage IA and 35% to 68% for stage IB),17 carmustine (CR rates of 86% for T1 and 47% for T2 disease),18 and bexarotene gel (CR rate of 21% in early-stage MF).19 Phototherapy with narrow-band ultraviolet B (NB-UVB) (54% CR in stages IA or IB)20 or psoralen + UVA (PUVA) (CR rate of 65%)21 is also frequently used. However, the most effective skin-directed therapy for MF is ionizing radiation.
Ionizing radiation is well suited to the treatment of cutaneous lymphoma. Lymphocytes are highly radiosensitive, and radiation doses may be effectively limited to the epidermis and dermis by appropriate selection of photon or electron energies. Options for irradiation include local superficial irradiation or irradiation of the entirety of the skin via total skin electron beam therapy (TSEBT).
FIGURE 79.3. Overall (A) and disease-specific (B) survival in mycosis fungoides by stage. (Reprinted with permission from Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome. Arch Dermatol 2003;139:857–866; copyright © 2003 American Medical Association. All rights reserved.)
Local Superficial Irradiation
A small portion (~5%) of patients with stage IA MF present with “minimal” disease, defined as a solitary lesion or two to three MF lesions clustered sufficiently close to one another that they are amenable to treatment with a single or abutting radiation fields.22 For these patients, treatment with local superficial irradiation may be considered. Treatment fields should be designed to encompass the entirety of the lesion (determined by visual inspection, palpation, and/or appropriate imaging) with a 1- to 2-cm margin, with use of a lead or Cerrobend cutout to conform field borders to the anatomy of the cutaneous lesion. Treatment is most commonly provided with electrons, with energies (usually 6 to 16 MeV) carefully selected to optimize dose penetration. The EORTC recommends that the 80% isodose line is set at the deep border of the dermis,23 which is commonly at a depth of approximately 4.5 mm.
Local superficial radiation is very effective in generating a CR for patients with “minimal” stage IA MF. In a review of 21 patients with “minimal” stage IA MF treated with local superficial radiation (superficial or orthovoltage x-rays or megavoltage electrons) to doses ranging from 20 to 40 Gy in four to five or 10 to 15 fractions, Wilson et al.22 found a CR rate of 97%. Importantly, review of the recurrence rates associated with different total doses of local superficial radiation revealed a 25% local recurrence rate (two of eight fields) with treatment to 20 Gy and an 8% local recurrence rate (two of 25 fields) with treatment to 20 to 40 Gy. Similarly, Cotter et al.24 reported a local recurrence rate of 42% for fields treated to 10 Gy or less, but 0% for fields treated to >30 Gy. More recently, treatment to 8 Gy in 4-Gy fractions has been shown to yield a CR rate of 92%.25 Based on these studies, it is recommended that “minimal” stage IA (i.e., unilesional or up to three closely approximated sites) MF lesions are treated to a dose of 30 to 36 Gy, and treatment as low as 8 Gy in two fractions may be considered as a palliative treatment. Side effects of local superficial radiation are usually limited to mild dermatitis, local alopecia, and pigmentation changes.
Total Skin Electron Beam Therapy
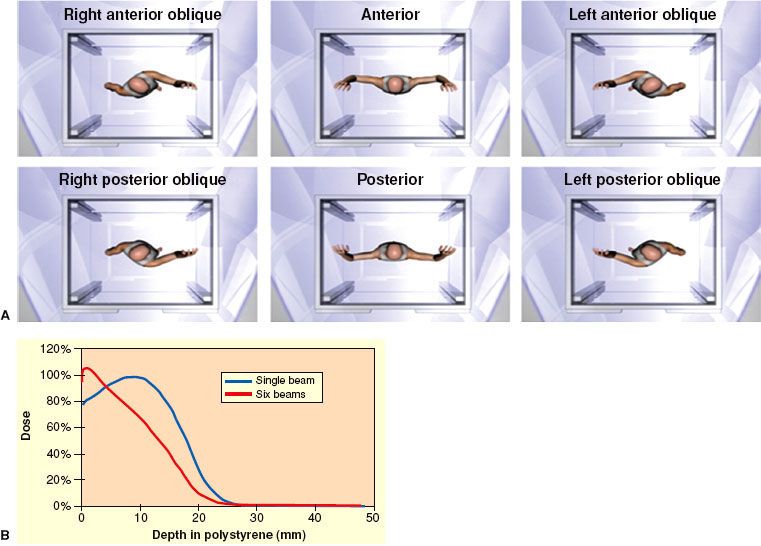
Technique. Total skin electron beam therapy (TSEBT) is technically challenging and should only be attempted in centers with special expertise in its provision, including skilled physics support. EORTC recommendations regarding the technical aspects of TSEBT are presented in Table 79.4. Modern TSEBT is usually accomplished with 6- to 9-MeV electrons generated by a medical linear accelerator directed at a patient standing behind a polycarbonate screen ~3.8 meters from the linear accelerator head. The polycarbonate screen scatters the incident electron beam and contributes to an improved surface dose. Treatment is provided in “cycles,” with one cycle composed of treatment of the patient in six different positions (Fig. 79.4A) over 2 days (three positions each day). Typically, a dose of 2 Gy is provided to the entirety of the skin during one cycle, and two cycles are usually administered per week. Treatment in six positions optimizes dose distribution at the skin surface (Fig. 79.4B). At Yale and Stanford, the anterior, right posterior oblique, and left posterior oblique positions are treated on cycle day 1, and the posterior, right anterior oblique, and left anterior oblique positions on cycle day 2.26,27 When the patient stands in a treatment position, a dual-field technique is used to deliver treatment to a superior and inferior field by angling the gantry 16 to 17.5 degrees above and below horizontal, respectively, the specific angle dependent upon individual machine characteristics (Fig. 79.5). Treatment to the six positions using the dual-field technique, which is in use at Yale and Stanford, delivers maximum dose to a depth of 1 mm, 80% dose to 6 to 7 mm, and 20% dose to 12.5 mm.26,27
FIGURE 79.4. A: The six total skin electron beam therapy (TSEBT) treatment positions as viewed from above. Images not to scale. B: Comparison of depth dose profiles associated with a single treatment position (blue curve) versus six treatment positions (red curve). (A courtesy of Christian Chang, www.christianchang.com, printed with permission from the artist. B from Smith BD, Wilson LD. Management of mycosis fungoides: Part 2. Treatment. Oncology 2003;17:1419–1428, with permission.)
Dose and Fractionation. Sublethal damage repair does not appear to be a major factor in determining the response of MF to ionizing radiation,28 and modern TSEBT is provided with a relatively protracted course of two cycles per week for 9 weeks, which provides a total dose of 36 Gy to the skin surface. The total radiation dose appears to be directly associated with complete response rates, with 18% CR with treatment to <10 Gy, 55% CR with treatment to 10 to 20 Gy, 66% CR with treatment to 20 to 25 Gy, 75% CR with treatment to 25 to 30 Gy, and 94% CR with treatment to 30 to 36 Gy.29 However, lower doses yield impressive rates of overall response (defined as a >50% reduction in cutaneous disease), overall survival, progression-free survival, and relapse-free survival rates. Relapse rates are relatively high even in patients in which a CR is obtained, and the absolute benefit of a CR relative to the increased side effects at higher doses of TSEBT is unclear. The application of reduced-dose TSEBT (10 to 20 Gy) in combination with additional therapies may be a viable alternative to the current standard of 36 Gy,30 but additional studies are necessary before any conclusions may be made in this regard.
Supplemental Treatments. The six treatment positions in TSEBT maximize unfolding of the skin and exposure of the skin surface to the incident electron beam, but areas such as the soles of the feet, perineum, and scalp remain obscured and require supplemental doses to ensure that a minimum of 20 to 28 Gy is administered to a depth of approximately 4 mm. Supplemental treatment to these areas may be accomplished by the use of 120-kV superficial photons with half-value layer (HVL) 4.2-mm Al or low-energy (~6 MeV) electrons with 1-cm bolus to treat the soles of the feet (1 Gy per fraction) and the perineum (1 Gy per fraction). In some setups, the scalp is treated by placing an angled electron reflector above the patient,26 but supplemental boosting is an alternative approach that is incorporated in some centers. Additional areas that may need supplemental dose include thick cutaneous tumors and skin folds secondary to body habitus, and the need for supplemental dose to such areas is based on the judgement of the radiation oncologist.26,27
Side Effects. In a review of perceptions of MF therapy, patients overall considered TSEBT to be a more difficult treatment to endure as compared to other treatments,31 and it is important that patients are advised that symptoms such as pruritus and cutaneous erythema may be exacerbated during therapy. Additional acute side effects that commonly occur include xerosis, dry desquamation, extremity edema, blister/bullae formation over the lower extremities, alopecia (including hair of the scalp, eyebrows, eyelashes, and body), and nail changes (nails may ultimately be lost but usually regrow). Hypohydrosis secondary to damage to sweat glands may occur. Similarly, dryness and irritation of the nasal mucosa may result in nose bleeds. Gynecomastia is a rare occurrence. Late/chronic side effects are minimal and include cataract formation, chronic xerosis, persistent alopecia, dystrophic nails, telangiectasia, and secondary skin cancers including squamous and basal cell carcinomas and melanomas.23,32–35
In an effort to minimize side effects, areas that are most susceptible to TSEBT such as the eyes, lips, hands, fingernails, feet, and testes are blocked during certain cycles of treatment. Shielding of the eye and lens may be accomplished by a combination of internal/external shields, selected based upon the proximity of clinical disease. Usually, if internal eyeshields are used, they are used for only a portion of the therapy (7 to 20 Gy). The lips, hands, and fingernails may be blocked by lead mitts or fingernail shields as clinical circumstances warrant. The feet may be blocked by footboards for a portion of the treatment. A testicular shield may be used during perineal boost treatments.26
FIGURE 79.5. Dual-field technique for total skin electron beam therapy (TSEBT). A: Treatment of superior field. B: Treatment of inferior field. Images not to scale. (Courtesy of Christian Chang, www.christianchang.com, printed with permission from the artist.)

Clinical Efficacy. TSEBT is very effective, particularly for early-stage disease. TSEBT yields a complete response rate of >90% with a 15-year relapse-free survival of 40% in patients with T1 disease, although it is no longer recommended for such limited disease.29 Of note, patients with recurrent disease after a course of TSEBT most commonly have disease restricted to <5% of the skin surface area, and these recurrences are therefore amenable to local salvage therapy with topical therapy or limited superficial radiation. When successful salvage of such limited recurrences is taken into account, relapse-free survival improves to 70% at 15 years.36–38
In T2 disease TSEBT is similarly effective, with a complete response rate of 76% to 90% and a 50% relapse-free survival rate at 5 years and 10% at 10 years. Relapse-free survival is significantly improved by addition of adjuvant PUVA or nitrogen mustard. Specifically, adjuvant PUVA improves 5-year relapse-free survival to 85%, and nitrogen mustard improves 10-year relapse-free survival to 40%.23,38–40
When cutaneous tumors are present (T3 disease), TSEBT is less effective but still yields an impressive complete response rate of 44% to 54% of patients, much greater than any other single modality. Adjuvant treatment should be considered, and retrospective studies suggest that nitrogen mustard may increase the durability of response. Alternatively, a combination of nitrogen mustard and local superficial radiation may be considered for tumors localized to a small percentage of the skin surface.38,40,41 Supplemental boosts should be considered for patients with tumors, and such boost treatment should be provided concomitantly with the initiation of TSEBT or prior to its initiation. The purpose of the boost is to diminish the thickness of the lesion so that electrons from TSEBT can effectively penetrate the entire lesion.
In erythrodermic MF (T4 disease), TSEBT yields a 70% to 100% response rate (for patients with T4N0 disease) and 5-year progression-free survival of 25% to 69%. When disease involves the peripheral circulation or extracutaneous sites, TSEBT is less effective and response and progression-free survival rates decrease to 74% and 36%, respectively. TSEBT appears to be synergistic with extracorporeal photopheresis (ECP), and the combination of TSEBT and ECP is associated with improved disease-specific survival and decreased levels of circulating malignant cells.42–45
TABLE 79.5 RECOMMENDATIONS FOR TREATMENT OF MF BY STAGE