Two prognostic scoring systems have been developed specifically for PCNSL.7,8 In a retrospective review of 105 PCNSL patients, the International Extranodal Lymphoma Study Group (IELSG) identified age >60 years, Eastern Cooperative Oncology Group (ECOG) performance status >1, elevated serum lactate dehydrogenase (LDH) level, elevated CSF protein concentration, and involvement of deep regions of the brain as independent predictors of poor prognosis. In patients with 0 to 1 factors, 2 to 3 factors, and 4 to 5 factors, the 2-year survival proportions were 80%, 48%, and 15%, respectively. In another prognostic model, PCNSL patients were divided into three groups based on age and performance status: (1) <50 years old, (2) ≥50 years old with a Karnofsky Performance Scale (KPS) ≥70, and (3) ≥50 years old with a KPS <70. Based on these three divisions, significant differences in overall and failure-free survival were observed.
There is no staging system that correlates with prognosis or response to treatment in PCNSL. However, because PCNSL is a multicompartmental disease potentially involving the brain, spinal cord, eyes, and CSF, the IPCG recommends an extent of disease evaluation, as noted previously, which will enable clinicians to follow the response to therapy.5
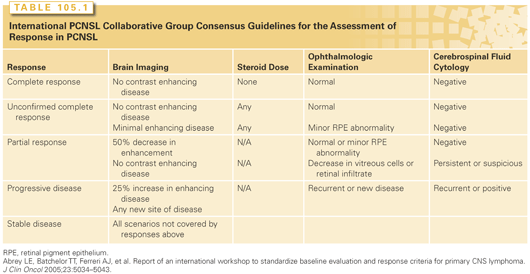
Defining a response to treatment in PCNSL requires an assessment of all sites involved by the disease. The IPCG has established response criteria that have been adopted into most prospective clinical trials (Table 105.1).5
Corticosteroids decrease tumor-associated edema and may result in partial radiographic regression of the tumor. An initial response to corticosteroids is associated with a favorable outcome in PCNSL.9 However, after an initial response to corticosteroids, almost all patients quickly relapse. Corticosteroids should be avoided if possible prior to a biopsy, given the risk of disrupting cellular morphology, resulting in a nondiagnostic pathologic specimen.
Surgical resection is not part of the standard treatment approach for PCNSL given the multifocal nature of this tumor.10 The role of neurosurgery in PCNSL is to establish a diagnosis via a stereotactic biopsy.
Standardized induction and consolidation treatment for PCNSL has yet to be defined. Historically, PCNSL was treated only with whole brain radiation (WBRT) at doses ranging from 36 to 45 Gy, which resulted not only in a high proportion of radiographic responses, but also in rapid relapse. In a multicenter, phase II trial, 41 patients were treated with WBRT to 40 Gy plus a 20 Gy tumor boost and achieved a median overall survival (OS) of only 12 months.11 Given the lack of durable responses to radiation and the risk of neurotoxicity associated with this modality of therapy, WBRT alone is no longer a recommended treatment for most patients with PCNSL. Moreover, because PCNSL is an infiltrative, multifocal disease, focal radiation or radiosurgery is not recommended. The most effective treatment for PCNSL is intravenous, high-dose methotrexate (HD-MTX) at variable doses (1 to 8 g/m2), typically utilized in combination with other chemotherapeutic agents and/or WBRT. However, there is no consensus on the optimal dose of HD-MTX or on the role of radiation in combination with methotrexate in the management of PCNSL. A number of randomized trials are ongoing to address these issues. Doses of methotrexate ≥3 g/m2 result in therapeutic concentrations in the brain parenchyma and CSF, and when combined with WBRT, lead to more durable treatment responses.12–14 In a phase II trial, 79 PCNSL patients were randomized to receive either HD-MTX (3.5 g/m2, day 1 or HD-MTX (3.5 g/m2, day 1) + cytarabine (2 g/m2 twice per day, days 2 to 3). Each chemotherapy cycle was 21 days. All patients underwent consolidative WBRT after induction chemotherapy. The HD-MTX + cytarabine arm had a higher proportion of complete radiographic responses and a superior 3-year OS.14 However, it is now widely recognized that there is a high incidence of neurotoxicity with combined modality treatment that includes WBRT.15 The latter observation prompted studies utilizing lower doses of WBRT. In a multicenter, phase II study, no significant neurocognitive decline was observed after consolidative reduced dose WBRT (23.4 Gy) and cytarabine in patients who had achieved a complete response to induction chemotherapy including HD-MTX.16 However, further study and longer neuropsychological follow-up of these patients is necessary to definitively assess the safety of this regimen because numerous studies have demonstrated the delayed neurotoxic effects of WBRT in the PCNSL population and the reduced risk of neurotoxicity in regimens consisting of chemotherapy alone.17,18 Given the risk of clinical neurotoxicity, other studies have assessed whether WBRT can be eliminated from the initial management of PCNSL. In a multicenter, phase III trial, patients were randomized to receive HD-MTX–based chemotherapy with or without WBRT.19 Five hundred and fifty-one patients were enrolled, of whom 318 were treated per protocol. The intent to treat analysis revealed that patients treated in the combined modality arm (chemotherapy + WBRT) achieved prolonged progression-free survival (PFS) but no improvement in OS, demonstrating that the elimination of WBRT from the treatment regimen did not compromise OS. This has led to deferral of WBRT and chemotherapy-alone approaches for newly diagnosed PCNSL patients. These approaches are based on a foundation of HD-MTX. Variable doses and schedules of HD-MTX have been utilized, but in general, doses ≥3 g/m2 delivered as an initial bolus followed by an infusion over 3 hours administered every 10 to 21 days is recommended for optimal outcomes and adequate CSF concentrations.20 Multiple, phase II studies have demonstrated the safety, efficacy, and relatively preserved cognition of HD-MTX–based chemotherapy regimens.21,22 Moreover, longer duration of induction chemotherapy with HD-MTX (>six cycles) results in higher complete response proportions.16,21
Several first-generation chemotherapy regimens for PCNSL included intrathecal chemotherapy. However, a number of nonrandomized studies that included intrathecal chemotherapy did not improve outcomes in PCNSL relative to regimens that did not include intrathecal injections of chemotherapy.23,24 Moreover, the ability to consistently achieve micromolar concentrations of MTX in the CSF at a dose of 8 g/m2 has led to the elimination of intrathecal chemotherapy from most of the chemotherapy regimens currently in use. However, the question regarding the role of intrathecal chemotherapy in the management of PCNSL should ultimately be addressed in a randomized trial.
Rituximab, a chimeric monoclonal antibody targeting the CD20 antigen on B lymphocytes, is being incorporated in combination regimens for PCNSL. When rituximab is administered intravenously at doses of 375 to 800 mg/m2, CSF levels from 0.1% to 4.4% of serum levels are achieved. Despite limited CSF penetration, radiographic responses have been observed in relapsed PCNSL patients treated with rituximab monotherapy, and this antibody has been incorporated into contemporary regimens for PCNSL.25 In a cooperative group, phase II study, 44 PCNSL patients were treated with induction chemotherapy consisting of HD-MTX at 8 g/m2 (day 1), rituximab at 375 mg/m2 (day 3), and temozolomide at 150 mg/m2 (days 7 through 11), all of which are drugs with demonstrated efficacy as monotherapy in PCNSL.22 This induction chemotherapy was followed by consolidation chemotherapy consisting of intravenous etoposide 5 mg/kg as a continuous infusion over 96 hours and cytarabine at 2 g/m2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








