Nation
Year approved
Reference
France
2003
[3]
Holland
2003
[4]
Germany
2010
[5]
Spain
2012
[6]
United Kingdom
2013
[7]
In the United States, the National Comprehensive Cancer Network guidelines have not as yet been changed to include CRS plus HIPEC for treating these three diseases. However, nearly all insurance companies in the United States authorize these treatments for their insured patients. Clearly, CRS plus HIPEC must be considered a treatment option in nations where modern cancer treatments are available.
7.3 Conceptual Changes that Contributed to the Progress of Cytoreductive Surgery and Perioperative Chemotherapy
Over the last three decades, oncologic, physiologic, and pharmacologic advances have contributed to the progress of CRS plus HIPEC treatment. No doubt, the initial success treating PMP was a “proof of principle,” in which the combination of complete CRS along with a perioperative chemotherapy lavage of peritoneal surfaces could, in a select group of patients, result in long-term survival, and even a cancer cure. Two observations in patients with AMN were crucial: First, these patients rarely developed metastases to lymph nodes or liver [8]. Second, even though there was a large volume of mucinous malignancy infiltrating diaphragm undersurfaces, the omentum, pelvic peritoneum, and small bowel was uninvolved or uninvolved to the extent that CRS combined with the perioperative chemotherapy lavage could maintain the small bowel in a disease-free state [9].
A second conceptual change in the surgical approach was the development of five different peritonectomy procedures [10]. The loose attachment of the parietal peritoneum allowed complete stripping of the peritoneum from the anterior abdominal wall, the right and left subphrenic spaces, the pelvis, and the omental bursa. Because the visceral peritoneum was more intimately attached to underlying structures such as stomach, small bowel, and large bowel, these peritonectomy procedures were of necessity combined with visceral resections in order to achieve complete CRS. As time went on, methodological refinements whereby cancer nodules were removed from the small bowel were published by Bijelic and Sugarbaker [11].
A third conceptual change involves the use of intraperitoneally administered chemotherapy. Pharmacologically, a new concept regarding a peritoneal-spaceto- plasma barrier was described and provided the rationale for intraperitoneal chemotherapy. The original pharmacologic principles regarding the movement of large molecules placed directly into the peritoneal space in a large volume of physiologic fluid were developed for the most part at the National Institutes of Health, Bethesda, MD, USA. Early publications by Flessner et al. in the experimental laboratory, and Myers and Collins and Speyer et al. in the clinic, suggested clinical utility of this new route of administration for cancer chemotherapy [12–14]. The importance of drug selection and proper dosimetry of intraperitoneal chemotherapy for vesicant drugs such as doxorubicin and for liver-metabolized drugs such as 5-fluorouracil (5-FU) was described by Sugarbaker et al. [15, 16]. The role of molecular size in maintaining this peritoneal-space-to-plasma barrier was clarified early on by Myers and colleagues [13].
Although little has changed over the course of the last three decades in the pharmacologic principles established by these early investigators, some clarifications of the use of chemotherapy within the peritoneal space have occurred [17]. First, it was made clear that the extent of peritonectomy had little to do with the continued presence of the peritoneal-space-to-plasma barrier. De Lima Vazquez et al. established that the percentage of parietal peritoneum removed had little or no impact on the pharmacology of intraperitoneal chemotherapy with 5-FU [18]. Second, the volume of intraperitoneal fluid used to dilute the chemotherapy solution and thereby fill the peritoneal space had an impact on the pharmacology of intraperitoneal drug instillation. Both Elias and Sideris and Sugarbaker et al. showed that a volume of intraperitoneal fluid had an impact on chemotherapy clearance into the body [19, 20]. If both volume and dose of chemotherapy were controlled, systemic exposure could be predicted, and intraperitoneal and systemic effects remained constant from patient to patient.
Perhaps the most clearly demonstrated clinical finding for successful treatment of peritoneal metastases is the absolute requirement for complete visible clearing of malignant disease from the peritoneal space for intraperitoneal chemotherapy to affect long-term survival [21].
The peritonectomy procedures were described initially by Sugarbaker in 1995 [10]. Yonemura and colleagues published similar procedures especially adapted for managing peritoneal metastases from GC [22]. Extensive visceral resections, including total gastrectomy, allowed surgical technology extension and the resulting optimal cytoreduction to a larger number of cancer patients [23].
Surgical technical advances associated with complete cytoreduction with peritonectomy involved the use of self-retaining retractors and ball-tip, highvoltage electrosurgery. A recent advance, results of which have not yet been completely realized, is the resurfacing of these extensive raw tissue surfaces with antisclerotic agents. Also needed are instructions at treatment centers in the advanced surgical technology required for complete CRS.
7.4 Early Postoperative Intraperitoneal Chemotherapy
The earliest reports of large numbers of patients with CRC and appendiceal malignancy showing long-term benefit from CRS combined with intraperitoneal chemotherapy were for treatment regimens using early postoperative intraperitoneal chemotherapy (EPIC) [21]. The most profound changes in the natural history of a peritoneal surface malignancy as a result of combined treatment seem to be in the minimally aggressive peritoneal surface malignancies, such as appendiceal cancer [24]. Elias and Pocard showed benefits from CRS with EPIC in CRC patients [25].
At the time of writing this chapter, EPIC remained the favored treatment plan for several chemotherapy agents when the intraperitoneal route of administration was favored. Drugs with a high rate of hepatic chemotherapeutic-agent extraction—so that a large proportion of the drug is detoxified with a single pass through the liver—are appropriate. These agents include 5-FU and doxorubicin [15, 26]. Also, taxanes, especially paclitaxel, are appropriate for EPIC. This drug is not significantly augmented by heat, works as a cell-cycle-specific drug that should be used over the long term, and is much better tolerated from the perspective of nausea and vomiting postadministration if given in divided doses over the first 5 days postoperatively. This drug has an area under the curve ratio of 1,000 and prolonged retention within the peritoneal space [27]. Clinical investigators are testing combinations of HIPEC and EPIC as a perioperative multidrug treatment plan, which may determine the optimal combination of these treatment strategies [28].
7.5 Heated Intraoperative Intraperitoneal Chemotherapy
The initial innovative efforts with heated intraoperative intraperitoneal chemotherapy (HIPEC) were by the efforts of Spratt et al. in 1980 [29]. Shortly thereafter, in 1988, Koga and colleagues at Tottori University, Japan, applied these treatments to patients with GC and peritoneal seeding [30]. Reports by Fujimoto et al. from Chiba University, Japan, and Yonemura et al. from Kanazawa University, Japan, should also be mentioned [31–34]. Studies from Japan involved GC patients with demonstrated peritoneal seeding or GC with adjuvant HIPEC.
Combining CRS with HIPEC was shown in a phase III trial to improve the survival of patients with CRC peritoneal seeding [35]. Also, a large retrospective multi-institutional study documented that in ∼ 25% of patients with CRC treated with this combined therapy will be alive and disease free at 5 years [36]. All natural history studies suggest that these patients have a median survival limited to ≤6 months [37–39].
7.6 Evolution of Prognostic Indicators Useful for Patient Selection
In the early efforts to manage carcinomatosis, patients were scored as carcinomatosis present versus carcinomatosis absent. In a group of patients with peritoneal seeding, no survival at 3 years was expected in patients with gastrointestinal cancer [37–39]. It became apparent that all patients with peritoneal metastases were not the same. Four different scoring systems by which to quantitate peritoneal metastases were described. Perhaps the original one was the “P factor,” utilized in the Japanese classification of GC: P1 (cancer seedlings limited to the stomach itself), P2 (cancer seedlings limited to the space above the transverse colon), and P3 (cancer seeding located throughout the peritoneal space) stood the test of time as a useful quantitation of gastric carcinomatosis [40]. For more precise quantitation of the distribution and extent of peritoneal metastases, the Peritoneal Cancer Index (PCI) was developed. This scoring system combines the distribution of peritoneal metastases and lesion size of nodules present throughout the abdomen and especially emphasizes cancerous involvement of the small bowel and its mesentery. The PCI can be scored preoperatively with a computed tomography (CT), at the time of abdominal and pelvic exploration, and after maximal efforts at cytoreduction have occurred [41]. Other methodologies for quantitating peritoneal cancer dissemination are the simplified PCI used at The Netherlands Cancer Institute and the Gilly Staging System from Lyon, France [35, 42].
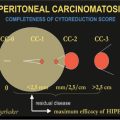
As more publications on peritoneal metastases appeared, an assessment of the completeness of cytoreduction was necessary. It was suggested that the Completeness of Cytoreduction (CC) score will vary as the invasive character of the malignancy and its response to neoadjuvant chemotherapy vary. A CC scoring system is reported [41].
It is obvious to those working long term in this field that early interventions in patients who have not had extensive prior surgery provides the best results in terms of survival and the lowest incidence of morbidity and mortality. Some means of assessing the extent of prior surgery was found to be necessary; thus, the Prior Surgical Score (PSS) was presented by Jacquet and colleagues and shown to have a major impact in determining survival of patients with appendiceal malignancy or ovarian cancer [24, 41, 43].
An essential adjunct to assessing prognosis in these patients is renewed interest in the histomorphology of peritoneal surface malignancy. The work of Ronnett and colleagues clearly shows that the invasive character of a malignant process, as estimated by histology, has a profound effect upon the success of combined treatment [44]. Similar emphasis on histomorphology in the outcome of combined treatment in patients with MPM was demonstrated by Cerruto et al. and Deraco et al. [45, 46].
7.7 Hyperthermia
Perioperative chemotherapy treatments over the last two decades have applied hyperthermia along with IP-CHT, with a presumed benefit. Hyperthermia in animal models increases drug cytotoxicity [47] and depth of chemotherapy penetration [48]; perhaps, if used long enough and at high enough temperatures, causes apoptosis from the heat itself. A single report, by Yonemura and colleagues, suggests that IP-CHT with heat is more effective than IP-CHT at body temperature [49]. Other studies confirming the benefits of hyperthermia have not been forthcoming. Also, studies show that EPIC is equivalent to HIPEC in maintaining a complete surgical response and improving long-term survival [50]. Certainly, in a patient who has undergone many hours of surgery with the abdomen and pelvis widely exposed often has moderate to profound hypothermia. The 90-min of hyperthermic lavage of the peritoneal space returns these patients to an optimal physiologic condition. In this regard, hyperthermia is an essential part of perioperative cancer treatment.
7.8 Peritoneal Surface Malignancy Treatment Centers
To the credit of Heald and Moran [7], the importance of a treatment center in the UK for patients with PMP was made clear and 1998 became a reality. Moran and colleagues [7] added greatly to the quality of care of patients with appendiceal malignancy in the UK. In 2002, a second center was established under the direction of O’Dwyer [7] and colleagues in Manchester, UK. Other designated treatment centers throughout Europe have since developed.
New efforts to further develop and improve outcomes of patients with peritoneal surface malignancy are underway. It is now clear that early treatment of minimal residual disease is optimal for these patients. Certainly, a watch-and-wait policy with referral of symptomatic patients to a peritoneal surface oncology center is no longer acceptable. Second, perioperative treatments are now many and varied. A bidirectional approach is becoming standard of care. As reviewed by van der Speeten and colleagues, some chemotherapy agents are most appropriate for intravenous use with heat targeting to the peritoneal cavity. Others are more valuable because of their large molecular size, and heat augmentation is used as part of the HIPEC regimen [51, 52].
Long-term intraperitoneal taxanes are now being explored, especially in Japan, for GC. The high response rate of combined systemic and intravenous application of chemotherapy reported by Yonemura et al. presents an exciting new treatment direction for patients with a very poor prognosis [53]. Kitayama et al. also continued applying adjuvant therapies for patients with peritoneal seeding using a combination of chemotherapy via an intraperitoneal port and systemic agents, which remains to be fully explored [54]. A summary of treatment evolution for patients with peritoneal metastases is shown in Table 7.2.
Table 7.2
Evolution of treatments for peritoneal carcinomatosis from gastrointestinal cancer (modified from [51])
Authors | Year | Event | Reference |
|---|---|---|---|
Spratt et al. | 1980 | Suggested a hyperthermic peritoneal perfusion system with the administration of intraperitoneal chemotherapy. University of Louisville, KY, USA | [29] |
Speyer et al. | 1981 | Pharmacology of intraperitoneal 5-fluorouracil in humans. National Institutes of Health, Bethesda, MD, USA | [14] |
Koga et al. | 1984 | Experimental study with prophylactic continuous hyperthermic peritoneal perfusion with mitomycin C. A significant prolongation of survival was obtained when 41.5°C hyperthermia was combined with mitomycin C. Tottori University, Japan | [55] |
Flessner et al. | 1985 | Pharmacokinetic studies established the peritoneal plasma barrier. National Institutes of Health, Bethesda, MD, USA | [12] |
Sugarbaker et al. | 1985 | Randomized controlled study of intravenous versus intraperitoneal 5-fluorouracil documented a diminished incidence of peritoneal carcinomatosis in patients with colon cancer. National Institutes of Health, Bethesda, MD, USA | [56] |
Koga et al. | 1988 | First study of adjuvant intraoperative hyperthermic peritoneal perfusion with mitomycin C in gastric cancer. Tottori University, Japan | [30] |
Fujimoto et al. | 1988 | Use of intraoperative hyperthermic peritoneal perfusion with mitomycin C combined with extended surgery in patients with gastric cancer and established peritoneal carcinomatosis. After treatment, 12.8% survived 1 year compared with 0% after surgery alone. Chiba University, Japan
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|