Key points
- 1.
Persistent expression of human papillomavirus (HPV) is required for progression to cancer.
- 2.
HPV vaccination has the potential to eradicate cervical cancer.
- 3.
Screening guidelines have changed dramatically with the use of contesting and increased intervals between screenings.
- 4.
The main screening objective is to allow for modified evaluation and treatment based on an individual woman’s risk.
- 5.
Self-collection HPV testing may increase screening by reaching those in traditionally under-screening areas or with other barriers to clinic-based screening.
Introduction
Worldwide, cervical cancer is a leading cause of cancer-related death and morbidity in women. In the United States, it is the third most common and lethal gynecologic malignancy. Despite the implementation of cytologic screening (Papanicolaou [Pap] testing) in the United States, it is estimated that in 2021 there will be over 14,000 new cases of cervical cancer, and almost 4000 cervical-cancer related deaths. The majority of cervical cancers are caused by persistent infection of the human papillomavirus (HPV). Women’s health providers must understand and comply with primary screening recommendations and management of abnormal screening results, particularly in ways that balance benefits and harms of screening and are cost-conscious.
Human papillomavirus natural history
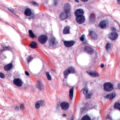
Over 99% of cervical cancers arise from persistent infection with HPV ( Fig. 1.1 ). HPV is an approximately 8000 base pair DNA virus, encoding eight major proteins. Approximately 200 strains of HPV have been identified, with 12 classified as carcinogenic (HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -59). Other types have been described as possibly carcinogenic (HPV-26, -53, -66, -67, -68, -70, -73, and -82). New genotypes are based on DNA sequencing and must share less than 90% DNA homology in the L1, E6, and E7 coding domains compared with existing HPV types.
HPV-16 is the most oncogenic, and perhaps the most clinically significant, genotype, and historically has accounted for over 50% of cervical cancers. HPV-18 is found in approximately 10% of cervical cancers, and is more frequently found in adenocarcinoma, which is commonly missed by Papanicolaou (Pap) testing. The other types are less oncogenic but have been reported in large typing studies of cervical cancers. HPV-18 and related HPV-45 are linked to cancers found in younger women.
Interactions of the HPV E6 and E7 gene products with p53 and pRb are critical for HPV-induced carcinogenesis. By inactivating or activating degradation of their targets, E6 and E7 eliminate genetic surveillance and allow unchecked cell cycling, leading to the accumulation of mutations and the potential for invasive cancer. HPV-16 E6 and E7 bind their targets with greater affinity than gene products of other HPV types which may partly explain its greater oncogenicity.
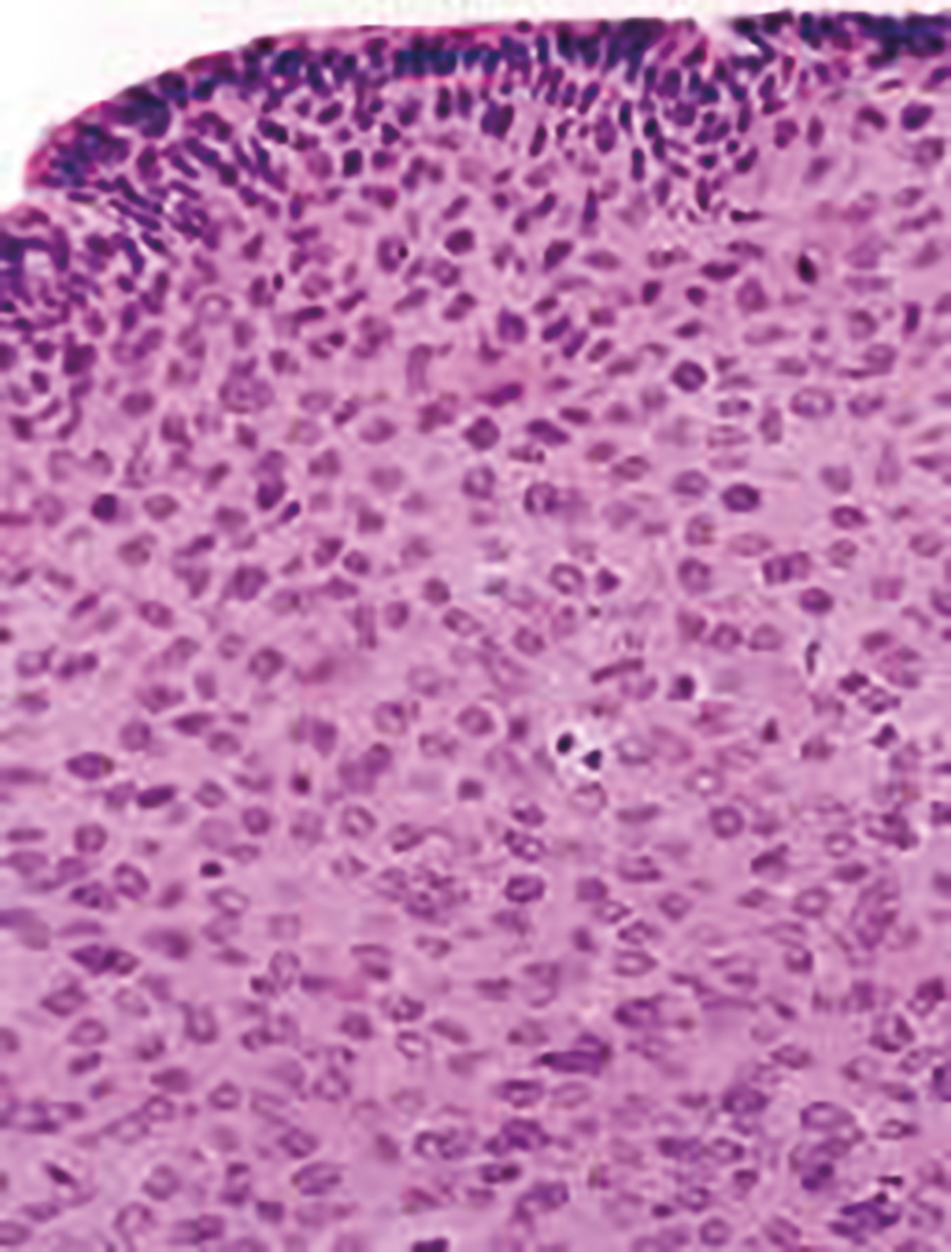
The progression from persistent HPV infection to cancer requires several key steps. First, infection of basal epithelial cells leads to establishment of a ring chromosome from which carcinogenic proteins are amplified while virion production occurs in maturing epithelium. Subsequently, disruption of the ring, often at the HPV E2 regulatory region, allows integration of E6 and E7 sequence into the host genome. The accumulation of mutations leads to nuclear changes visible cytologically as high-grade squamous intraepithelial lesions (HSILs) and histologically as high-grade cervical intraepithelial neoplasia (CIN) ( Fig. 1.2 ). Selection for invasiveness and metastasis through additional mutations and gene methylation ultimately results in the development of cancer. Notably, preexisting HPV infections do not appear to predispose to or protect from infection by unrelated types.
While lifetime abstinence protects against genital HPV infection, nonpenetrative sexual behaviors may still transmit the virus, and additional male exposures increase female risk. For example, spouses of men who engaged in sex with prostitutes were at higher risk for cervical cancer than those of men who did not, and cervical cancer risk is higher among women whose husbands had more sexual partners. Women who report recent sex only with women are also at risk, though their risk may be lower than that of heterosexual women. Condom use is not fully protective against HPV infection because condoms fail to cover wide areas of genital skin, though it speeds clearance of HPV infections. Male circumcision also reduces but does not eliminate HPV and cancer risks. For these reasons, all women with prior sexual experience, including those who have not been sexually active for years, remain at risk for cervical cancer and require screening until they meet criteria for screening exit as discussed later.
While the percentage of women who at some point develop HPV infection is high, most women infected with carcinogenic HPV do not develop cervical cancer. The majority of infections are cleared immunologically, and women who clear the virus are at low risk for progression to cancer or reappearance of CIN2+. HPV is an intraepithelial virus, and clearance appears to require recognition of infection by cell-mediated immune cells. Roughly half of new infections are cleared within 6 months, with half of the remainder cleared by the end of the first year after infection. Clearance is associated with greater density of CD8+ cells and lower density of T-regulatory cells in underlying stroma. Cervical treatment speeds clearance and reduces risk for posttreatment acquisition of new HPV infections. The type distribution of HPV infection after hysterectomy shows that HPV-16 and HPV-18 have a greater predilection for cervical rather than vaginal epithelium, with HPV types of lesser oncogenicity dominating in the post-hysterectomy vagina. Women infected at an increased age do not typically develop preinvasive disease or cancer of the vagina; however, increased age may result in immune senescence which suggests that some HPV infections in older women derive from reactivation of previously acquired latent infections.
Although determinants of HPV persistence and progression of HPV infection to invasive cancer are poorly understood, several risk factors are known. HPV infection of a cervix undergoing active metaplasia increases risk, as reflected by the epidemiologic observations that early onset of first intercourse is associated with cancer. Smoking is linked to both CIN and cervical cancer. Benzopyrenes have been identified in cervical mucus, and the interaction of tobacco carcinogens with carcinogenic HPV increases risk substantially. Smoking also reduces immune-mediated HPV clearance. Cervical adenocarcinoma and adenocarcinoma in situ (AIS) have been linked to oral contraceptive use. Variants of common HPV types that segregate by ethnicity and polymorphisms in genes related to HPV immune recognition or HPV protein products also modulate HPV persistence and carcinogenic progression. Perhaps most important, lack of screening is a high-risk factor for progression of HPV infection to precancer and cancer. Appropriately screened and managed women with multiple risk factors are at relatively low risk, and women with few risk factors who are not screened are at higher risk.
Immune factors play a clear role in the clearance or persistence of HPV-related cervical lesions, but the nature of immune defects is poorly understood. Immunosuppression related to coinfection with the human immunodeficiency virus (HIV-1) illustrates the importance of immunity in the typical control of HPV. Women with HIV have much higher rates of HPV infection, including multitype infections. HPV clearance rates are lower, although most women do clear their HPV infections if observed long enough, especially if immune reserve as measured by CD4 lymphocyte count remains above 200 cells/mm 3 . Although most HPV infections in HIV-seropositive women are cleared to such low levels of viral expression that they become nondetectable even with sensitive assays, reactivation appears to occur. This is apparent in cohort studies as the reappearance of previously cleared infections in women who deny sexual activity, often because of illness. Risks in other immunosuppressed states appear to be similar.
HPV infection predicts risk for subsequent CIN2+, even among cytologically normal women. In most cases, persistent HPV infections result first in cytologically detectable abnormalities and then in colposcopically visible lesions that grow before developing into invasive cancers. The 10-year risk of CIN2+ after a single detected HPV infection exceeds 10%. As developed by Richart through observational studies of the cervix using cytology and colpomicroscopy, a diagnosis of CIN was based on progressively severe nuclear aneuploidy, abnormal mitotic figures, and loss of epithelial maturation. Initially considered a progressive lesion, CIN was thought to begin as a small lesion with atypia near the basement membrane of the cervical transformation zone, gradually increasing in size and becoming less differentiated with an increasing proportion of the epithelium taken up by atypical cells until a full-thickness carcinoma in situ developed and then became invasive. Given this concept of progression from low-grade to CIN2+ disease to cancer, lesions of all grades were treated. When progression does occur, however, it appears to require years. HPV acquisition commonly follows sexual debut, the median age being approximately 17 in the United States, but the peak age of cervical cancer diagnosis lags by some 3 decades. This long transition time allows for even moderately sensitive screening tests to identify persistent lesions for treatment before invasive cancer develops.
Gradually, the regressive nature of most low- and midgrade lesions becomes apparent. Low-grade lesions, which include genital warts and CIN1, are histologic expressions of HPV infection and are thus not considered to be premalignant. Greenberg and associates found that of 163 women with CIN1 after low-grade cytology followed for a median of 36 months, 49% regressed, 43% persisted, and only 8% progressed to CIN3. In the Atypical Squamous Cells of Undetermined Significance/Low Grade Squamous Intraepithelial Lesion Triage Study (ALTS), a large randomized trial of management options for women with borderline cytology results conducted under the auspices of the US National Cancer Institute (NCI), 2-year risk for CIN3 was 10% among women with CIN1. As reported by Castle et al., after controlling for HPV genotype, with HPV-16–associated CIN1 progressing to CIN3 in 19% of cases, biopsy-proven CIN1 was not a risk factor for progression. However, these risk estimates may be substantially higher for women with prior CIN2+ cytology.
CIN2 lesions or greater (CIN2+) appear to represent clonal lesions arising from single-type HPV infections. Although women may harbor multiple HPV types in the genital tract, most multitype infections are associated with multifocal lesions. Moscicki et al. showed that 63% of adolescents and young women with CIN2 lesions had resolution without treatment within 2 years; subsequent clearance was minimal, rising only to 68% after an additional year. McAllum et al. showed a similar 62% regression after only 8 months of observation for women with CIN2 younger than 25 years of age. No patients in either study progressed to cancer during observation. In both studies, CIN2 likely represented recent HPV infections, not true premalignant lesions. Regression rates are lower in older women, at least in part because lesions detected later may have been persistent for years, and lesions that have evolved mechanisms to evade host immune-mediated clearance are likely to continue to persist. Castle et al. compared CIN2 rates in the immediate colposcopy and cytology surveillance arms of the ALTS. They found that over 2 years, approximately 40% of CIN2 regressed. Trimble and colleagues showed that HPV-16–associated lesions are less likely to resolve. Their finding of associations with human leukocyte antigen (HLA) alleles and regression support a role for HLA-restricted HPV-specific immune responses in determining clearance.
Untreated, CIN3 poses considerable risk of progression to invasive cancer. An observational study of women in New Zealand with CIN3 diagnosed between 1955 and 1976 were observed; among 143 women reported by McCredie et al. managed only by punch or wedge biopsy, 31 progressed to cancer of the cervix or vagina after 30 years. Risk rose to 59% in 92 women with persistent disease after 2 years of observation. These findings show both that treatment of CIN3 should be strongly considered regardless of age or other factors, but also that not all CIN3 lesions will inevitably progress to cancer.
Even treated CIN3 continues to pose a risk of progression to cancer. Women in the New Zealand study whose treatment appeared adequate by current standards faced only 0.7% cancer risk after 30 years. Studies from Scandinavian countries with integrated health systems can link databases on procedures and subsequent cancers and provide accurate long-term results with minimal loss to follow-up. Strander et al. showed that risk for cervical cancer rose significantly in previously treated women after age 50 years, with standardized incidence ratios compared with untreated women ranging from 3 to 5. Vaginal cancer risks were elevated across all ages, although the absolute risk of vaginal cancer was low. In Finland, Kalliala et al. confirmed this long-term increased risk and also found an elevated risk for nongenital smoking-related cancers. Jakobsson et al. found that in addition to cervical cancer, women treated for CIN faced higher mortality rates from cardiovascular disease, alcohol-related, and traumatic death, consistent with the demographic and behavioral factors linked to CIN.
Epidemiology
Approximately 20 million Americans and 630 million persons worldwide are infected with HPV. In the United States, about 6.2 million people will acquire a new infection annually. More than 80% of sexually active individuals acquire genital HPV infections. Prevalence rates are highest among women in their late teens and early 20s. Risk factors for HPV acquisition include smoking, oral contraceptive use, and new male partners.
Among high-risk HPV types, HPV-53 is most common, detected in 5.8% of US women ages 14 to 59 years screened in the National Health and Nutrition Examination Survey (NHANES) between 2003 and 2006. This was followed by HPV-16 (4.7%), HPV-51 (4.1%), HPV-52 (3.6%), and HPV-66 (3.4%). HPV-18 was present in only 1.8% of screened women. In this study, demographic risk factors for prevalent HPV infection included younger age (peaking at ages 20 to 24 years), non-Hispanic black ethnicity, non-married status, non-college educated, and living below the poverty line. Behavioral risk factors included reporting ever having sex, age of first intercourse before 16 years, increased number of lifetime partners, and number of partners in the past year.
HPV infection determines subsequent risk for precancer. Among women enrolled in a Portland health maintenance organization who had HPV-16, the 10-year risk for CIN3, AIS, or cancer was more than 15% after HPV-16 infection, almost 15% after HPV-18 infection, less than 3% after other oncogenic HPV infections, and less than 1% after a negative HPV test result. A Danish study of over 8000 women found that infection with, and especially persistence of, HPV-16/18/31/33, was associated with high absolute risks of development of CIN3+. Additionally, in a study by Elfgren et al., the authors demonstrated persistence of HPV infection at 1- and 3-year intervals following initial screening was predictive of CIN2+.
It is estimated that there were approximately 14,000 new cases of cervical cancer in the United States in 2020. However, the annual incidence of CIN2+ is approximately 6 to 10 times higher than cervical cancer incidence. Preinvasive lesions begin to appear on average 2 years after infection. Cancer risk is quite low soon after infection; despite a high prevalence of HPV among sexually active teens, cervical cancer incidence is only about 1 in 1,000,000 before 20 years of age. Among women who develop CIN2+, only 30% to 50% will develop cancer over years of observation.
Clear risks for CIN and cervical cancer have been identified. The international Collaboration of Epidemiological Studies of Cervical Cancer reviewed evidence for various risk factors for cervical cancer and carcinoma in situ (though their studies were not linked to HPV data). They found that oral contraceptive use raised the risk for cervical disease by 1.9-fold for every 5 years of use. First intercourse before 15 years of age was associated with twice the risk of cervical cancer found in women with first intercourse after 23 years of age, and having more than five lifetime sexual partners carried more than double the cervical cancer risk of lifetime monogamy. Lesser but still significant increases in risk were associated with number of pregnancies and earlier age at first term pregnancy. Both squamous cancers and adenocarcinomas share epidemiologic risk factors, except that smoking is linked only to squamous cancers. The most notable risk factor for the development of invasive cervical cancer, however, is infection with and persistence of HPV, particularly HPV-16, which is found in nearly 50% of cases.
The role of family history in determining cervical cancer risk is complex. Dissociating genetic components of familial risk from cultural ones is difficult, as sexual attitudes and behaviors, reproductive patterns, and smoking are often linked to family. Zelmanowicz et al. assessed the role of family history in cohorts of women prospectively studied in Costa Rica and the United States. A family history of cervical cancer in a first-degree relative tripled the risk for CIN3 or squamous cervical cancer. The effect persisted after controlling for HPV exposure. No effect of family history on adenocarcinoma risk was seen. Although several genome-wide association studies (GWASs) have identified a range of genetic variants in candidate pathways that might contribute to cervical oncogenesis, Chen et al. in a large Chinese GWAS found that only HLA and major histocompatibility class I polypeptide-related sequence A genes were identified as candidate risk genes across several populations.
Lower socioeconomic status (SES) and minority ethnicity are also linked to CIN and cervical cancer risk in the United States, although distinguishing cultural contributions to cervical cancer risk, such as a sense of fatalism, distrust of the medical care system providing screening services, and lack of health education about the benefits of screening, are difficult to distinguish from biologic risks related to ethnicity and SES, such as genetic predisposition, toxin exposure, and micronutrient deficiencies.
Human papillomavirus vaccination
HPV vaccination has the potential to eliminate cervical cancer, given HPV causes the vast majority of cases. Intramuscular delivery of synthetic HPV L1 capsid antigens results in humor immunity; current vaccines are created in protein synthesis using cell culture systems. No actual live or killed virions are used, and therefore HPV vaccines cannot cause HPV-related cancer. Despite early concerns that humoral immunity would be insufficient to prevent infection, vaccine efficacy in preventing type-specific HPV persistence approaches 100%. Currently available vaccines are largely prophylactic. However, a recent meta-analysis demonstrated that patients who underwent surgical excision followed by HPV vaccination were less likely to develop recurrent CIN.
Three HPV vaccines have been used in the United States. The quadrivalent HPV vaccine (available 2006–17) protected against HPV-16 and -18, which together account for almost 70% of all cervical cancers, as well as HPV-6 and -11, which are the most common causes of genital warts. The benefit of cervical cancer prevention, which might take decades to become manifest, is augmented by its ability to prevent genital warts, a concern for many young women. The bivalent HPV vaccine protects against only HPV-16 and -18. It is no longer available for use in the United States and may have superior antigenicity and may have some cross-protection against HPV types related to HPV-16 and -18. The nonavalent vaccine (available since 2017) is effective against the same types as the quadrivalent vaccine and also includes coverage against HPV types 31, 33, 45, 52, and 58; enhanced coverage should prevent 90% of all cervical cancers and many other HPV-related cancers at other sites.
Ideally, because HPV vaccines are prophylactic, population-based vaccination should begin before the initiation of sexual activity. Because 5% of US 13-year-old girls are sexually active, the target age for HPV vaccination is the ages of 11 to 12 years. However, vaccination can be initiated at 9 years of age. The vaccination schedule is dependent on patient age. For patients younger than 15, two doses of the vaccine should be administered, with the second administration between 6 and 12 months following the initial. Because teen sexual activity is unpredictable, delaying vaccination until girls are more mature risks missing the vaccination window for many. Nevertheless, many sexually active young women show no evidence of infection by target HPV types, and “catch-up” vaccination up to 26 years of age should be considered. Vaccination through age 45 years has been approved but appears cost-ineffective with minimal population-level cancer prevention impact. Testing of cervicovaginal secretions and serum antibody testing are both insensitive for detecting prior HPV vaccination and are not recommended before a decision about HPV vaccination. After age 15, three doses of the vaccine should be administered (at 0, 1 to 2, and 6 months). These vaccination strategies are endorsed by the CDC.
Several countries have instituted organized vaccination programs, either mandatory or using a school-based opt-in mechanism with high uptake. Countries that used quadrivalent vaccine have documented a dramatic decrease in genital warts among teens but not older women, and abnormal cytology rates have also fallen in the youngest women.
In the United States, vaccination rates are suboptimal. Data in 2019 demonstrated that in adolescents aged 13 to 17, 71.5% had started the vaccine series and nearly 54.2% had completed it. However, this is still well below the Healthy People 2030 goal of 80%. Regrettably, despite the potential for vaccination to eliminate the disparately high risk of cervical cancer among women of minority ethnicity and lower SES, uptake has been lowest in these groups, potentially widening cancer disparities in future years. Work by the CDC’s HPV-IMPACT group has demonstrated vaccine effectiveness of 1, 2, and 3 doses of the HPV vaccine and falling rates of CIN2+ lesions positive for HPV-16 and -18, particularly in the youngest women receiving the vaccine.
Risks of vaccination are low and the most commonly cited adverse effects are tolerable. These include fever, rash, injection site pain, nausea, headache, and dizziness. Anaphylactic and vagal reactions may be fatal, so vaccination should only be administered in sites with ability to manage anaphylaxis and fainting. Despite initial concerns, HPV vaccination status does not enter into young women’s decisions to initiate sexual activity. Vaccination is contraindicated for pregnant women, although no congenital anomalies or adverse pregnancy outcomes have been linked to HPV vaccination, and the series may begin after delivery. Interruption of vaccination does not appear to require re-initiation of the three-shot series. The duration of vaccine effectiveness is unclear, but antibody levels remain elevated for several years after vaccination.
Presently, history of HPV vaccination does not alter screening recommendations for US women, but this is expected to change in the future. This is because many women of screening age were not vaccinated before initiating intercourse, so vaccine effectiveness is unclear. There is no central US vaccine registry, and identifying vaccinated women by self-report may be inaccurate. No HPV vaccine covers all carcinogenic HPV types, so women vaccinated before first intercourse remain at risk for infection and cancer due to strains not covered by the vaccine.
In October of 2018, the Food and Drug Administration (FDA) extended the approval of the nonavalent HPV vaccine to previously unvaccinated individuals up to age 45 for men and women for the prevention of all HPV-related cervical, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers. Previous approval extended only through age 26. However, modeled studies have shown that reductions in cases of CIN2+ and cervical cancer by extending vaccine approval are extremely modest while significantly increasing costs.
Screening
The goal of any cancer prevention program is the reduction of morbidity and mortality through intervention prior to the onset of symptoms. For cervical cancer, the current mechanism to achieve this goal is the identification and destruction of CIN2+ lesions that are presumed precancers.
Classically, screening has relied on Papanicolaou cytology testing followed by colposcopic assessment of women with Pap abnormalities, directed biopsies, and treatment of biopsy proven CIN2+ lesions. However, Papanicolaou testing is relatively insensitive, and a single Pap test may be negative in almost half of women with CIN2+. Simultaneously, progression from HPV infection to cancer usually requires several years, allowing for multiple rounds of screening, with greater sensitivity than single tests.
Cytologic interpretation includes all mutations, methylations, and other genetic modifications that alter the nuclear and cytoplasmic appearance of cells. To be clinically useful, these changes must be categorized in a manner that reflect a common natural history. Papanicolaou developed a five-class grading system, from normal to invasive cancer, with atypia, dysplasia, and carcinoma in situ between. To unify terminology, the NCI convened a consensus meeting that developed the 1988 terminology known as the Bethesda System for cervicovaginal cytologic diagnosis. The Bethesda System has been revised multiple times, most recently in 2014. It identifies cytology as satisfactory or unsatisfactory, includes nonneoplastic changes, and divides epithelial cell abnormalities into squamous and glandular changes of varying degrees of severity ( Table 1.1 ). Distinguishing squamous from glandular abnormalities is critical because glandular abnormalities carry much higher risk for CIN2+, including squamous abnormalities, as well as endometrial cancer and cervical adenocarcinoma and AIS. Squamous changes related to HPV are termed “squamous intraepithelial lesions (SILs)” because some lesser changes do not reflect dysplasia or neoplasia, only cytomorphologic changes of HPV infection. Indeterminate lesions are termed “atypical squamous cells (ASCs),” and these are subdivided into ASC “of undetermined significance (ASC-US),” which carries a low risk of associated CIN2+, or “cannot exclude high-grade SIL (ASC-H),” which is a more worrisome finding that requires immediate colposcopy. An online atlas allows pathologists to standardize findings and interpretations against national norms ( https://screening.iarc.fr/atlascyto.php ).
|
Most cytologic tests in the United States are conducted using liquid-based assays. In these tests, cells are collected and suspended in preservative solution and then transferred to a slide. Liquid-based cytology was marketed as more sensitive than conventional Pap tests. However, a meta-analysis by Arbyn et al. showed that, although liquid-based cytology yields more abnormalities, including CIN2+, it is not superior to conventional smears in cancer prevention. Nevertheless, it remains preferred in the United States primarily because it allows for molecular triage of equivocal results using HPV and other assays. Interpretation is still done visually, although some centers use automated imaging and pattern recognition software to eliminate the least abnormal slides. Cytotechnologists perform initial assessment, with slides containing abnormal findings and a proportion of normal slides read by cytopathologists.
The effectiveness of screening has not been demonstrated in randomized trials, but population studies have shown clear benefits. An NCI study by Erickson et al. assessed cytology screening by vaginal aspiration for 108,000 women in Shelby County, Tennessee. They showed a high yield of unsuspected CIN2+ and early cancer at the first screen, with a substantial reduction in invasive lesions in the second screen. Gustafsson et al. reviewed data from 17 cancer registries and showed marked effects, especially in Scandinavian countries. Eddy assessed the impact of screening on cervical cancer incidence and death. Without screening, a 20-year-old average-risk woman faces a 2.5% risk of cancer and a 1.2% risk of cancer death. Triennial screening between ages 20 and 75 years reduces risk to less than 0.4% and 0.1%. Annual screening does improve effectiveness, but by less than 5% and with a notable increase in cost.
Initially, screening was opportunistic, and women underwent screening when they presented for care, which was generally at annual visits. This screening strategy remains the norm in the United States, although recommended screening intervals have lengthened; some electronic medical records prompt clinicians when screening is due; and some health care organizations have developed standards, rewards, and reminders. Several countries with centralized medical care systems have developed organized screening, with coordinated identification, invitation, and management of women due for screening (though this is not the case in the United States). Serraino et al. showed that the move from opportunistic to organized screening in Italy resulted in a decline in cervical cancer incidence and a downstaging of incident cervical cancers after an increase in precursor detection. Additionally, Quinn et al. found that a national call/recall system with incentive payments to general practitioners in Britain instituted in 1988 increased screening coverage to 85% of the target population, increased detection of CIN2+, and reduced mortality in women younger than 55.
Despite its overall benefit, cytology-based screening has several weaknesses. Most fundamentally, the process of screening, triage, and treatment is cumbersome, and noncompliance, which can be a significant problem, at any point renders it ineffective. Cytology results are reported in ways that can be confusing, and efficient, effective management may require integration of current results with prior abnormalities. Costs of combined HPV testing with cytology are increased compared to cytologic testing alone. Multiple studies have shown that most women who develop cervical cancer in developed countries, especially those presenting at advanced stages, are inadequately screened. Sung et al. studied incident cancers in a US prepaid health plan and found that 53% were nonadherent to screening, 28% had false-negative Pap tests, 4% had inadequate follow-up after an abnormal Pap test result, and the rest either developed cancer despite appropriate investigations or were unclassified. Kinney et al. in the same US health maintenance organization found that 60% of cervical cancer patients were inadequately screened. Deeper exploration of the records of long-standing plan members with inadequate screening showed that 70% had missed opportunities for screening in primary care clinics.
In addition, cytology-based screening performs poorly in younger women. Sasieni et al. showed that cervical screening in women ages 20 to 24 years had little impact on actual cancer risk until those women reached 30 years of age, but screening older women results in an immediate benefit. Because younger women have low rates of cervical cancer incidence and death but high rates of HPV infection, abnormal cytology, and CIN destined to regress, the benefits of early initiation of screening may be difficult to balance against potential harms. Cytology preferentially detects squamous cell carcinomas, and the impact of cytology screening on adenocarcinoma incidence has been muted.
Over 99% of all cervical cancer is caused by HPV. The incorporation of HPV testing into screening has allowed for the implementation of longer screening intervals. As HPV assays have been developed, protocols have utilized HPV testing as a primary screening test as well as in combination with cytology (co-testing) and as a triage test for women with borderline cytology results. Only high-risk HPV types have a role in screening; because low-risk types essentially play no role in identification of HPV infection in the absence of visible genital warts, testing for low-risk types is contraindicated. HPV genotyping assays for types 16 and 18 identifies women at higher risk. The disadvantage of HPV testing is its lower specificity, with up to 30% of young women testing positive in some studies. Because all commercially available HPV tests have a detection threshold designed to balance sensitivity and specificity, a negative test result does not exclude low-level HPV infection, and prior cytologic or histologic abnormalities may mandate close follow-up or even treatment despite absence of detectable HPV. HPV tests should not be collected in media whose effects of test performance have not been evaluated by the FDA.
Screening does have potential harms. Identification of HPV infection, abnormal cytology, and cervical cancer precursors is not without consequences, including anxiety, relationship disruption after diagnosis of a sexually transmitted infection, the inconvenience and cost of accelerated follow-up visits, and the pain of repeated examinations. Treatment of precursor lesions also carries risks, including bleeding, infection, and injury to adjacent organs. Some studies have suggested that destructive cervical treatments increase the risk for preterm delivery and pregnancy loss. US studies have failed to replicate these results in women after cervical loop electrosurgical excision procedure (LEEP). Women with CIN2+ are at higher risk for pregnancy loss than those who do not, perhaps because of common risk factors, including smoking, nutritional deficiencies, and lower SES, and these confounding factors may account for differences. However, there may be a threshold effect for treatment, and women with deep or repeated excisional procedures may be at higher risk for pregnancy loss. Harms of excessive screening are limited, and include stigmatization, unfounded fear of cancer, and the potential of undergoing interventions without cancer prevention benefit. Sharp et al. showed that depression, distress, and anxiety occurred in 15% to 30% of women in the months after reporting of marginal cytology abnormalities.
After the utility of screening is accepted, societies, women at risk, and clinicians must decide when to initiate screening, which screening tests to use, how often to screen, and when toward the end of life the identification of asymptomatic disease ceases to be beneficial. With all choices, sensitivity and specificity must be balanced. Earlier screening starts with more sensitive tests at shorter intervals until later in life will decrease cancer incidence and mortality, but costs and harms from diagnosing lesions that would never have progressed to cancer will increase. In developed societies, guidelines for screening have been developed by experts assessing evidence for benefit and harm and deciding how these can best be balanced. In 2012, guidelines were released by the US Preventive Services Task Force (USPSTF) and a consensus conference sponsored by the American Cancer Society (ACS), the American Society for Colposcopy and Cervical Pathology (ASCCP), and the American Society for Clinical Pathology (ASCP) ( Table 1.2 ). Guidelines for screening recommended by the USPSTF were updated in 2018 and those by the ACS were updated in 2020.