Key points
- 1.
Ovarian cancer is the most lethal of all gynecologic cancers. Elderly women are more likely to develop ovarian cancer than are young women.
- 2.
Oral contraceptives decrease the incidence of ovarian cancer substantially.
- 3.
Ovarian cancer may be inherited, with increased risk being caused by a mutation in BRCA1 or BRCA2 , mismatch repair defect, or any number of other less common genes tested in the current panels.
- 4.
A combination of surgery and chemotherapy is almost always recommended for treatment. The order depends on the extent of disease, volume, and location.
- 5.
Poly (ADP-ribose) polymerase (PARP) inhibitors and bevacizumab may be incorporated as maintenance and treatment across the entire ovarian cancer continuum.
- 6.
Significant strides have been made in survival as treatment and surgery have evolved.
Malignant neoplasms of the ovary are the cause of more deaths than any other female genital tract cancer. In the United States, there were approximately 21,750 new cases and 13,940 deaths in 2020 as a result of ovarian cancer. Ovarian cancer accounts for 5% of all cancer mortality among women. In the United States, deaths from this cause occur at a rate of one every 44 minutes, and this disease will develop in one of every 68 women. Doctors and patients alike continue to be frustrated by our lack of understanding of the factors that lead to ovarian cancer and the failure to achieve a significant reduction in mortality.
Classification
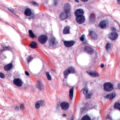
The most practical classification scheme is based on the histogenesis of the normal ovary, shown in Table 9.1 . The early development of the ovary is divided into four stages. During the first stage, undifferentiated germ cells (primordial germ cells) become segregated and migrate to the genital ridges, which are bilateral thickenings of coelomic epithelium. The second stage consists of proliferation of the coelomic epithelium and the underlying mesenchyme. During the third stage, the ovary becomes divided into a peripheral cortex and a central medulla. The fourth stage is characterized by the development of the cortex and involution of the medulla. The histology classification categorizes ovarian neoplasms with regard to their derivation from coelomic epithelium (epithelial ovarian cancer), germ cells (germ cell tumors), and mesenchyme (sex cord-stromal tumors).
|
The majority (85% to 90%) of malignant ovarian tumors are epithelial. They can be grouped into predominant histologic types as follows:
| Serous cystadenocarcinoma | 42% |
| Mucinous cystadenocarcinoma | 12% |
| Endometrioid carcinoma | 15% |
| Undifferentiated carcinoma | 17% |
| Clear cell carcinoma | 6% |
| Carcinosarcoma | 1% |
The specific malignant histologic type has less prognostic significance than clinical stage, extent of residual disease, and histologic grade. However, histologic grade is an important independent prognostic factor in patients with epithelial tumors of the ovary ( Fig. 9.1 ). Because survival for patients with grade II and III tumors is more closely related, research studies frequently stratify by low-grade (grade I) and high-grade (grade II and III) tumors.

Incidence, epidemiology, and etiology
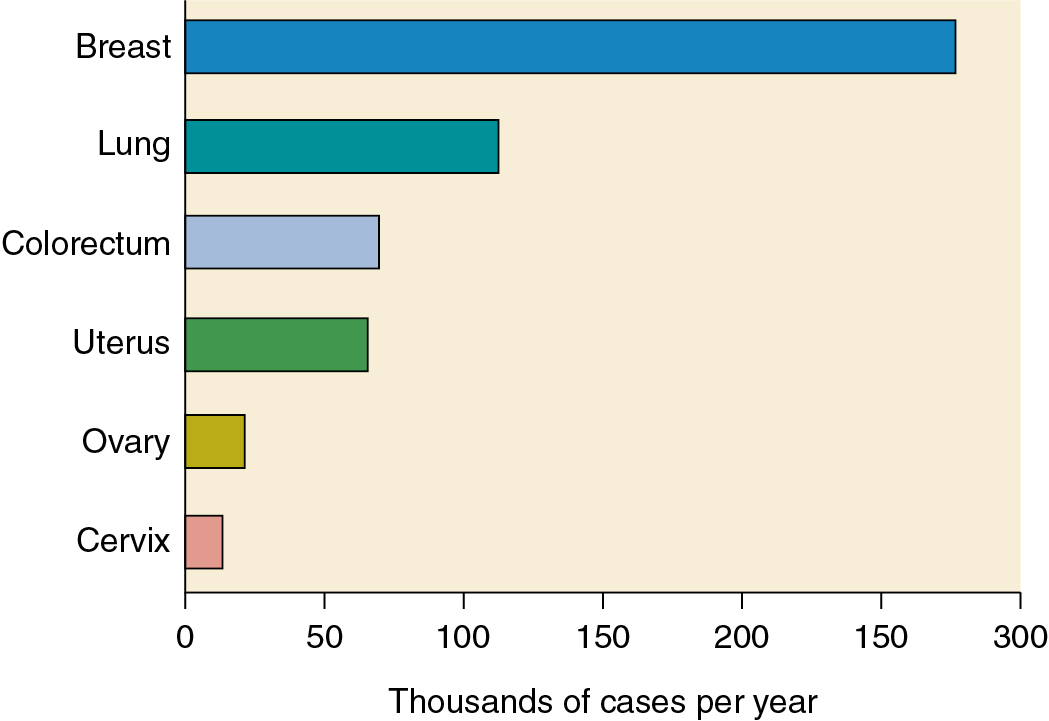
Approximately 19% of gynecologic cancers are of ovarian origin ( Fig. 9.2 ), but 42% of all gynecologic cancer deaths occur in women who have ovarian cancer. Cancer of the ovaries is the fifth most frequently occurring fatal cancer in women in the United States and ranks high as a cause of cancer death in other developed nations. Ovarian cancer will develop in approximately 11.2 per 100,000 women per year in the United States ( Table 9.2 ), and the death rate is 6.7 per 100,000 women per year. Death is often attributed to repeated bouts of intestinal obstruction as the tumor spreads over the surface of the bowel and patients develop inanition and malnutrition and frequently have a steady increase in symptom burden as they approach the end of life. However, since the 1970s, both incidence and mortality rates have declined. Among women older than 65, incidence and mortality rates initially increased until 1990 but have declined since that time. These changes may have resulted from increased use of oral contraceptives in younger patients and a shifting of the survival curve to the right. When matched for stage, survival outcomes are worse in older women. In some cases, this may be a result of less aggressive treatment in the older woman and higher percentage of low-grade disease in younger patients. Since 2006, death rates have declined for non-Hispanic White women, Black women, Hispanic women, and Asian and Pacific Islander women but have remained relatively stable in Native American/American Indian and Alaskan native women.
| Site | Probability (%) |
|---|---|
| All sites | 39.5 a |
| Breast | 12.9 |
| Cervix | 0.6 |
| Colorectal | 4.2 a |
| Lung | 6.3 a |
| Ovary | 1.2 |
| Pancreas | 1.6 |
| Uterine corpus | 3.1 |
Malignant neoplasms of the ovaries occur at all ages, including infancy and childhood ( Fig. 9.3 ). Throughout childhood and adolescence, the rate of death from ovarian carcinoma in the United States is exceeded by those for leukemia, lymphomas, and neoplasms of the central nervous system, kidney, connective tissue, and bone. The major histologic types occur in distinctive age ranges ( Table 9.3 ). Malignant germ cell tumors are most commonly seen in girls younger than age 20 years, whereas epithelial cancers of the ovary are primarily seen in women older than age 50 years. At age 40, the 10-year probability of developing ovarian cancer is 1 in 870 for women in the United States. At age 50, this increases to 1 in 474, and age 80 is approximately 1 in 283. The largest absolute number of patients with ovarian cancer is found in the age group 60 to 64 years. Indeed, more than one-third of the cases occur in patients 65 years or older. Elderly women are more likely than younger women to be in advanced stages of ovarian cancer at initial diagnosis, and the 5-year relative survival rate for elderly women is approximately half the rate (28.4%) observed in women younger than 65 years (56.6%).

| Type | Age <20 Years (%) | Age 20–50 Years (%) | Age >50 Years (%) |
|---|---|---|---|
| Coelomic epithelium | 29 | 71 | 81 |
| Germ cell | 59 | 14 | 6 |
| Specialized gonadal stroma | 8 | 5 | 4 |
| Nonspecific mesenchyme | 4 | 10 | 9 |
A decreased incidence of ovarian cancer is related to less frequent ovulation. These observations coincided with data illustrating a reduction in the disease incidence with the use of oral contraceptive pill (OCP) use . The hypothesis that OCP use reduced the risk of epithelial ovarian cancer was suggested in 1979, and studies have demonstrated that use of OCP, compared with nonusers, may reduce the risk of ovarian cancer by 40% in women aged 20 to 54 years. This benefit was noted with even a few months of OCP use, and the reduction in risk appeared to persist for as long as 10 years after use has ceased. The theory of “incessant ovulation” suggests that the epithelial lining of the ovary may be sensitive to the events of ovulation, which in turn can act as a promoting factor in the carcinogenic process. Gross and Schlesselman determined the effect of oral contraceptive use on the cumulative incidence of epithelial ovarian cancer from ages 20 to 40 years, 20 to 50 years, and 20 to 55 years and stratified these age categories by family history and parity. The number of epithelial ovarian cancer cases estimated to occur per 100,000 oral contraceptive users decreased with increasing duration of use. Their results suggest that 5 years of use by nulliparous women can reduce their ovarian cancer risk to the level seen in parous women who never use oral contraceptives, and 10 years of use by women with a positive family history can reduce their risk to a level less than that of women whose family history is negative and who never use oral contraceptives. On the basis of these data, the recommendation is strongly made that premenopausal patients with risk factors for ovarian cancer use oral contraceptives when pregnancy is not being sought.
Over many years, studies have disagreed as to the association between fertility drug exposure and ovarian cancer. Early reports noted that infertile women treated with fertility drugs had a 2.8 times increased risk of ovarian cancer compared with women without infertility, and the risk was higher among women who did not become pregnant compared with the parous women. However, specific drug exposure was not documented. Subsequent studies disagreed as to whether induction with clomiphene or gonadotropins were associated with increased risk. A subsequent meta-analysis including 10 case-control or cohort studies revealed no obvious association between assisted reproductive technologies and ovarian cancer. The published data suggest that there is probably little relationship between fertility drug use and ovarian cancer. Further investigations are underway to clarify this association. The concept is appealing when one considers the reduction in incidence noted with the use of oral contraceptives and parity.
Consistent with this theory of incessant ovulation, a population-based study noted protection against ovarian cancer with increasing parity. One study noted that in a study of 197 women with ovarian cancer, nulligravida were 2.5 times more likely to have malignant ovarian tumors and 2.9 times more likely to have an ovarian tumor of low potential malignancy than were women who had been pregnant three or more times. One possible interpretation could be that the endocrine state of pregnancy protects against ovarian cancer and that lack of this protection places infertile women at higher risk for ovarian cancer. A second explanation could be that infertility and ovarian cancer result from a similar genetically abnormal gonadal status. This theory would explain why infertile women are more at risk than never-married and never-pregnant women. Parazzini and colleagues studied the influence of various menstrual factors on risk of epithelial ovarian cancer. They reported that the risk of ovarian cancer rose with later age at menopause and with early menarche. In addition, Danforth and coworkers prospectively examined risk of ovarian cancer in the Nurses’ Health Studies and found that women who never breastfed faced a 1.5-fold risk of ovarian cancer compared with women who breastfed for longer than 18 months. These associations further support the “incessant ovulation” theory.
A number of other modifiable and nonmodifiable risk factors for ovarian cancer have been explored. Epidemiologic evidence strongly suggests that environmental factors play a major role in developing epithelial cancer of the human ovary. Highly industrial countries have the highest reported incidence, and physical or chemical products of industry may be causative factors. A notable exception is highly industrialized Japan, where rates for ovarian cancer have been among the lowest recorded in the world. Of interest, the incidence of ovarian cancer increases in Japanese immigrants to the United States and their offspring, eventually approaching that of Anglo-Saxon White women by the second and third generations. This suggests that causative carcinogens may be present in the immediate environment, such as food, behavioral habits, and other influences that change gradually during the cultural transition.
Studies of dietary fat have been inconclusive, and other dietary factors are not well characterized. Although the Women’s Health Initiative investigators reported low-fat diet was associated with up to a 40% reduction in ovarian cancer risk, several large cohort studies have not been able to confirm this finding. Because diet and exercise are potentially modifiable risk factors, continued investigation into their role is warranted.
Some have suggested that ovarian cancer may be initiated by a chemical carcinogen through the vagina, uterus, and fallopian tubes and that damaged ovarian surface epithelium from ovulation induces inflammation and mutation susceptibility. Certainly, many different chemical substances could migrate from the vulvovaginal areas to the upper genitalia and be implicated in carcinogenesis. The agent most extensively studied has been talc powder, but many of the studies that noted a slight increased risk of ovarian carcinoma in association with talc did not adjust for other factors (oral contraceptive use, family history) that are related to ovarian cancer. Others included low malignant potential tumors with their invasive cancers or did not correct for integrity of the female genital tract status. The relationship (if any) of talc to ovarian cancer would appear to be minimal at best.
No epidemiologic or experimental evidence exists to incriminate viruses in the development of neoplasms of the human ovary. Attempts to isolate viruses from cultures of human ovarian cancer cells have been unsuccessful to date. Because of its gonadotropic properties, mumps virus is an obvious candidate among known viruses for oncogenic activity in the ovary. However, the evidence for mumps virus as an etiologic agent in ovarian cancer currently remains speculative.
Familial ovarian cancer
Familial hereditary ovarian cancer currently falls into three categories:
- 1.
Site-specific familial ovarian cancer
- 2.
Hereditary breast-ovarian cancer syndrome, in which there is an increased incidence of breast and ovarian carcinomas alone or in combination
- 3.
Lynch syndrome type II, in which family members may develop a variety of cancers, including colorectal, endometrial, and ovarian cancer
Inherited genetic mutations are associated with approximately 15% of women who develop ovarian cancer. The mutation is inherited in an autosomal-dominant fashion (maternal or paternal transmission), and multiple family members are affected over several generations. First-degree relatives (mother or sister) are frequently involved.
True hereditary ovarian and breast cancer mainly result from mutations of BRCA1 and BRCA2 genes. BRCA1 mutations are located on the long arm of chromosome 17q, and BRCA2 mutations are located on chromosome 13q12. Initial evaluation of these germline mutations suggested an estimated risk of ovarian cancer of 39% to 46% for carriers of the BRCA1 mutation but a lower rate of 10% to 20% for carriers of the BRCA2 mutation. With more than 400 mutations identified in the BRCA1 and BRCA2 genes along with multiple polymorphisms, estimating risk is difficult. Mutations in more rare DNA damage repair genes, such as BRIP1, RAD51C , and RAD51D , can also contribute to ovarian cancer risk. Individuals with these mutations generally have a germline mutation (inherited mutated copy of the gene) in contrast to somatic mutations (noninherited or acquired). Common germline mutations are listed in Table 9.4 . Lynch II syndrome arises as a result of inherited mutation in a family of DNA repair genes (MSH2, MSH6, MLH1, PMS2); this group accounts for only a small number of inherited ovarian cancers. In most women with a strong family history who develop ovarian cancer, the disease is sporadic in nature and not inherited.
a Increased risk; management based on family history (insufficient evidence for risk reducing surgery recommendations).
In general, it is estimated that 10% to 15% of patients with ovarian cancer in the general population are carriers of a breast or ovarian cancer susceptibility gene. The risks for ovarian cancer in carriers to the age of 60 and 80 years were estimated to be approximately 10% and 27%, respectively. Carriers were predicted to have at least a 15-fold age-specific risk of ovarian cancer compared with noncarriers. Some ethnic groups appear to be at high risk for gene mutation, particularly the Ashkenazi Jewish population. In a large study of 5318 Jewish subjects, 120 women were identified as BRCA1 and BRCA2 carriers. Carriers had a significantly elevated risk, with an estimated risk of 2% by age 50 years and 16% by age 70 years compared with the noncarrier risk of 0.4% by 50 years and 1.6% by 70 years.
Kerlikowske and associates reviewed published studies of familial ovarian cancers, preceding BRCA testing, using ovarian cancer incidence data from the Surveillance, Epidemiology, and End Results (SEER) database to estimate lifetime probabilities of ovarian cancer in these families. They noted that the lifetime probability of ovarian cancer for a 35-year-old woman was 1.6% in an individual without a family history of ovarian cancer. It increased to 5% if she had one relative and 7% if she had two relatives with ovarian cancer.
In considering women at high risk, two questions should be addressed: (1) Who should be tested? and (2) Are there screening or prophylactic measures that can be applied to decrease the risk? A family history of breast cancer, particularly before the age of 50 years, or ovarian cancer at any age in a first-order relative, and Ashkenazi Jewish ancestry, are high-risk factors for BRCA1 or BRCA2 germline mutations. Family history, both maternal and paternal, should be obtained (three generations is desired), and age at diagnosis should be noted. Ideally, pathology reports or death certificates verify accuracy of history because there can be considerable errors from history alone. Lifetime empirical risks can be determined from this information and may suggest that testing for a gene mutation is indicated. Extensive evaluation of family history and counseling should be done before genetic testing. Interpretation and plan of action that will influence medical management should be discussed in detail before genetic testing is performed. Testing of family members known to have cancer before testing of the cancer-free members is recommended. If a suspect mutation is found in the family member with cancer, the relatives without cancer can be tested for that specific mutation, which is known as cascade testing. If the family member with cancer does not have a mutation, other relatives probably will not benefit from testing.
Risk-reducing salpingo-oophorectomy (RRSO) after completion of childbearing, or age 35 to 45 years, in women at high genetic risk is associated with a significant reduction in the risk of BRCA1- or BRCA2 -associated ovarian cancer. A National Institutes of Health (NIH) Consensus Conference recommended that women with two or more first-degree relatives with ovarian carcinoma be offered RRSO after completion of childbearing or age 35 years (BRCA1) or 45 years (BRCA2). RRSO does not prevent subsequent primary peritoneal cancer, in which failure rates are 4% to 5% at 20 years after RRSO. Additional risk reduction estimates include that RRSO was associated with an 80% risk reduction in BRCA1- or BRCA2 -associated ovarian, fallopian tube, and peritoneal cancer and a 50% risk reduction in BRCA1- or BRCA2 -associated breast cancer. Although a benefit on survival has not been demonstrated, the National Comprehensive Cancer Network (NCCN) states that in women who do not desire RRSO, transvaginal ultrasound and cancer antigen (CA) 125 levels can be performed on an individualized basis starting at age 30 to 35 years. Both of these tests carry relatively high false-positive and false-negative rates with low positive predictive value (PPV) and have not been shown to impact overall mortality. However, similarly to the general at-risk population, the use of oral contraceptives protected against ovarian cancer in patients with mutation in either the BRCA1 or BRCA2 gene.
A number of national organizations, including the Society of Gynecologic Oncologists (SGO) and the American Society of Clinical Oncology (ASCO), have recommended all women diagnosed with epithelial ovarian, tubal, and peritoneal cancers receive genetic counseling and be offered genetic testing, with consideration for multigene panel testing, even in absence of a family history. This testing should include somatic BRCA testing in the tumor given the implications for subsequent therapy.
The Gynecologic Oncology Group (GOG) conducted a study (GOG 199) of 2605 women at increased risk for developing ovarian cancer, either by known BRCA mutation or family history risk factors. Patients were enrolled after choosing either prophylactic oophorectomy or screening, and both groups followed with CA 125 levels and Risk of Ovarian Cancer Algorithm (ROCA) assessment. The study was designed to evaluate both the prospective incidence of new and occult ovarian cancers and to assess the ROCA and quality of life. In 2014, Sherman and colleagues reported a portion of the analysis of high-risk women older than 30 years of age who elected RRSO. Clinically occult cancers were detected in 2.6% of high-risk women ( BRCA1 mutation carriers, 4.6%; BRCA2 mutations carriers, 3.5%; noncarriers, 0.5%), and further results from this trial will be forthcoming.
Signs and symptoms
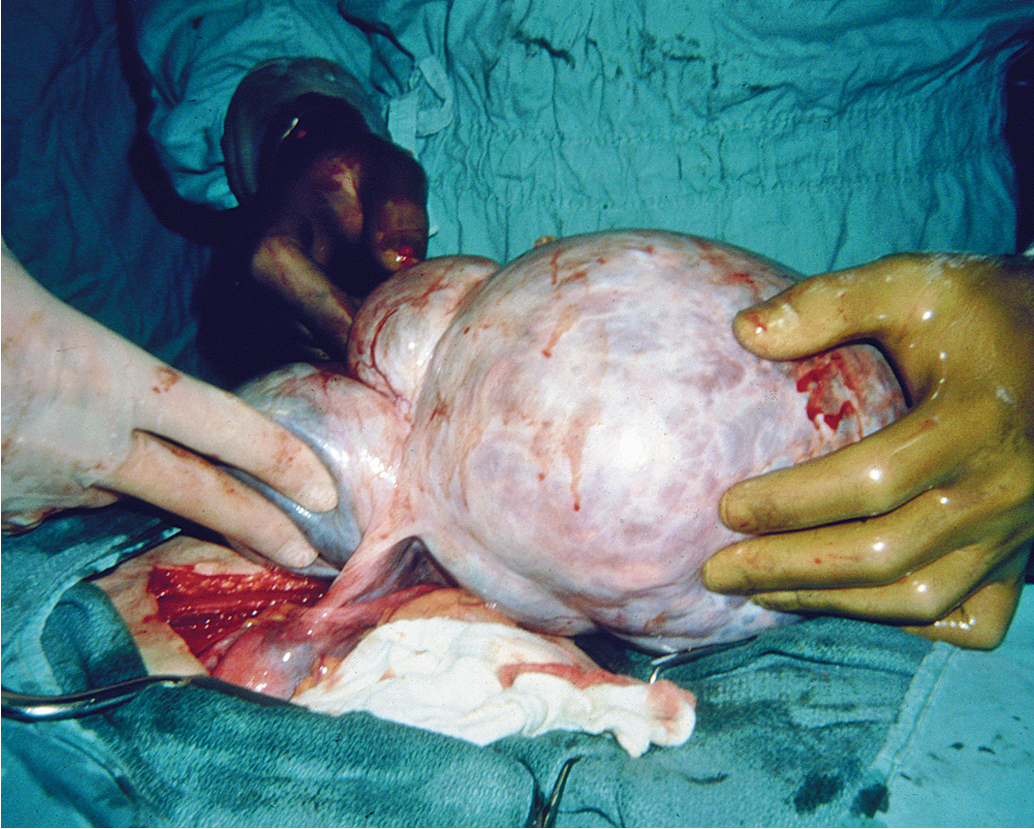
Although the wide range of ovarian tumor types generally presents in a similar manner, the diagnosis of early ovarian cancer is often more a matter of chance than a triumph of the scientific method. As the ovary enlarges ( Fig. 9.4 ), there may be progressive compression of the surrounding pelvic structures, producing vague abdominal discomfort, urinary frequency, and “pelvic pressure” ( Table 9.5 ). The insidious onset of ovarian cancer needs no elaboration. As the neoplasm reaches a diameter of 10 to 15 cm, it may account for abdominal enlargement. The notions that the development of ovarian cancer is “silent” and there are no early symptoms of ovarian cancer are a focus of the patient advocacy “listen for the whisper” educational efforts. This is in part due to the fact that pain and abdominal distention are usually late complications, typically seen only in early disease with a complication such as torsion, rupture, or, rarely, infection. Other symptoms may include abdominal bloating, early satiety, and/or urinary frequency/urgency, and these vague symptoms may be present for several months before the diagnosis. The majority of such nonspecific complaints are not alarming in themselves, resulting in overlooking the possibility of ovarian cancer in many cases; however, almost 90% of patients noted symptoms prior to diagnosis. If no other symptoms develop, it is only when the patient has gross abdominal enlargement marking the occurrence of ascites and abdominal carcinomatosis when appropriate diagnostic evaluation is undertaken. Therefore a high index of suspicion is warranted in all women between the ages of 40 and 69 years who have persistent abdominal symptoms that cannot be diagnosed.
| Symptom | Odds Ratio |
|---|---|
| Abdominal/pelvic pain swelling | 19.1 |
| Abdominal swelling | 11.2 |
| Urinary frequency | 5.3 |
| Unable to eat/feeling full | 1.0 |
A persistent ovarian enlargement should be an indication to consider surgery, with the diagnosis confirmed by pathology. The size of the tumor does not indicate the severity of disease; indeed, many of the largest neoplasms are histologically benign, most commonly mucinous cystadenoma. Moreover, many large adnexal masses may be of nonovarian etiology. Nonovarian causes of apparent adnexal masses are diverticulitis, carcinoma of the cecum or sigmoid, pelvic kidney, and uterine myomas ( Table 9.6 ). Therefore thorough evaluation and final pathology are required to make the definitive diagnosis of ovarian cancer. Routine pelvic examinations detect only one ovarian cancer in 10,000 asymptomatic women. However, pelvic examination remains the most practical means of detecting early disease. The physician should have a high index of suspicion for an early ovarian neoplasm in any ovary palpated in a menopausal patient. These patients should be evaluated with additional imaging and considered for laparoscopy or laparotomy when ultrasound findings suggest malignant change (e.g., complex mass, >5 cm, or intracystic papillations).
| Benign | Malignant | |
|---|---|---|
| Surface papilla | Rare | Very common |
| Intracystic papilla | Uncommon | Very common |
| Solid areas | Rare | Very common |
| Bilaterality | Rare | Common |
| Adhesions | Uncommon | Common |
| Ascites (100 mL) | Rare | Common |
| Necrosis | Rare | Common |
| Peritoneal implants | Rare | Common |
| Capsule intact | Common | Infrequent |
| Totally cystic | Common | Rare |
Diagnostic techniques
Although signs and symptoms through history and physical examination may raise suspicions for ovarian cancer, further diagnostic testing, tumor markers, and imaging will aid in risk assessment and referral to gynecologic oncologist for additional surgical management. The role of tumor markers may be used as a tool to assist diagnosis of ovarian cancer. CA 125 is an antigenic determinant of a murine immunoglobulin Ig1 monoclonal antibody and is expressed in more than 80% of nonmucinous epithelial ovarian cancers. Traces of the antigen are expressed in adult tissues derived from the coelomic epithelium, including mesothelial cells lining the pleura, pericardium, and peritoneum, and from the epithelial component of the female reproductive system. Thus there are other causes of elevated CA 125 levels, which include both gynecologic and nongynecologic conditions. Acute inflammatory processes involving the pelvic area and other regions of the body may increase CA 125 ( Table 9.7 ). If a cut-off of 35 U/mL is chosen for the upper limit of normal, an elevated CA 125 level should be found in 1% of apparently normal blood bank donors, 6% of patients who have benign disease, 28% of individuals who have nongynecologic malignant neoplasms, and 82% of patients who have surgically demonstrable epithelial ovarian cancer. Therefore, although not diagnostic, CA 125 levels may heighten one’s suspicion of ovarian cancer.
| Gynecologic | Nongynecologic |
|---|---|
| Acute pelvic inflammatory disease | Active hepatitis |
| Adenomyosis | Acute pancreatitis |
| Benign ovarian neoplasm | Chronic liver disease |
| Endometriosis | Cirrhosis |
| Functional ovarian cyst | Colitis |
| Meigs syndrome | Congestive heart failure |
| Menstruation | Diabetes (poorly controlled) |
| Ovarian hyperstimulation | Diverticulitis |
| Unexplained infertility | Mesothelioma |
| Uterine myoma | Nonmalignant ascites |
| Pericarditis | |
| Pneumonia | |
| Polyarteritis nodosa | |
| Postoperative period | |
| Renal disease | |
| Rodent exposure (HAMA response) | |
| Systemic lupus erythematosus |
Another tumor marker that may guide management is a CA 19-9 level. Although it is important to rule out pancreatic tumors, CA 19-9 may be elevated in mucinous ovarian tumors/cancers. Carcinoembryonic antigen (CEA) should also be obtained to rule out the possibility of a primary gastrointestinal (GI) tumor. In the setting of an elevated CEA or the presence of specific GI symptoms, additional evaluation with a colonoscopy and/or upper GI endoscopy should be conducted. In general, a ratio of CA 125 to CEA of 25:1 or greater is usually suggestive of an ovarian cancer diagnosis and can be used as diagnostic support for neoadjuvant chemotherapy with positive cytology.
Imaging also plays a significant role in the detection of ovarian cancer. Often pelvic ultrasound (transvaginal or transabdominal) is used to evaluate abnormal symptoms or findings on pelvic examination. Findings that may increase the suspicion of ovarian cancer include solid components such as nodularity or papillations, thick septations, and ascites; the sensitivity of ultrasound in the assessment of ovarian cancer approaches 90%, and specificity ranges from 68% to 83%. Computed tomography (CT) with contrast enhancement may be helpful in identifying the extent of clinical disease. Chest imaging should be considered in patients with advanced disease or those with respiratory symptoms. Magnetic resonance imaging (MRI) is generally not used for evaluation but may be considered to further evaluate etiology of a mass and/or if CT contrast allergy is present. However, no imaging modality is conclusive, and suspicious pelvic masses, especially in postmenopausal women, should be evaluated surgically.
In general, if surgical intervention is feasible, we recommend against needle biopsy of ovarian masses in the absence of extraovarian pelvic disease because such a procedure can result in spillage of malignant cells into the peritoneal cavity. Regardless of whether the fluid contains neoplastic cells, surgery will likely still be necessary to remove either the large benign neoplasm or to define the extent of the malignant process. In patients with symptomatic ascites or pleural effusion, aspiration of fluid may be therapeutic as well as diagnostic and guide the management plan (see neoadjuvant chemotherapy later). However, it is important to note that up to 50% of ascitic fluid samples from patients with true ovarian malignant neoplasms will be negative for malignant cells on cell block analysis.
At the time of surgery, it may be difficult to discern the malignant potential of a particular ovarian neoplasm. There is sufficient overlap of morphologic criteria to cause confusion, and the error rate of frozen section ranges from 5% to 15%; therefore the diagnosis rests with the final histologic examination. In the event that ovarian cancer is unexpectedly encountered at the time of surgery, intraoperative gynecologic oncology consultation should be obtained. If not readily available, biopsy of a metastatic lesion (e.g., peritoneal) can be obtained if able to be done safely and the remainder of the procedure should be terminated.
To help with evaluating a patient with a complex pelvic mass, the American College of Obstetricians and Gynecologists (ACOG), in conjunction with the SGO, published recommendations that could help to direct referral to or consultation with a gynecologic oncologist. Physicians should perform a pelvic examination and imaging as appropriate for the patient’s symptoms or physical examination findings. For premenopausal women with a suspicious pelvic mass, referral to a gynecologic oncologist should be considered by the presence of at least one of the following: significantly elevated CA 125 level, ascites, abdominal or distant metastases, or one or more first-degree relatives with breast or ovarian cancer. For postmenopausal women with a concerning pelvic mass, consultation should be considered for any CA 125 elevation, ascites, nodularity or limited mobility, evidence of metastasis, or a first-degree relative with breast or ovarian cancer.
Attempts at early detection (screening)
Screening studies have been conducted in average risk women. Jacobs and colleagues studied 22,000 postmenopausal women screened with CA 125 determinations and have reported follow-up observations for a mean of 6.76 years; 767 women (3.5%) had elevated CA 125 values, of whom 49 (6%, or 0.0022% of the total screened) had cancer. One-third of these were early stage. Overall specificity was 97%, and sensitivity at 1 year and 7 years of follow-up was 75% and 57%, respectively. For a low-incidence cancer such as ovarian cancer, cost-effective screening strategies require a PPV greater than 10%. This generally requires a technique specificity of greater than 99%, with a high sensitivity of greater than 75%. In this study, and others, the PPV of 3% was not high enough to be an effective screening method, and there is no current role for screening with CA 125 levels in unselected women.
The value of ultrasonography in screening for early ovarian carcinoma has received much attention. In a study by van Nagell and colleagues, more than 46,000 postmenopausal or high-risk women were prospectively evaluated with annual ultrasound. The investigators detected a total of 71 invasive ovarian cancers and 17 borderline tumors. Of the invasive cancer cases, 42% were detected in stage I, 21% in stage 2, and 37% in stage 3. Disease-specific survival was improved when compared with historical controls; however, the study included higher-risk women, and there was no direct comparison. One potential pitfall of ultrasound evaluation is the focus on the ovaries that may miss multifocal or extraovarian disease at earlier recognizable states, and further evaluations are ongoing.
Screening attempts have also been conducted using multimodality evaluations including the use of transvaginal ultrasound and CA 125 levels, and several large studies have taken on this task. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial randomized participants to annual transvaginal ultrasound and CA 125 levels ( n = 34,253) or usual care ( n = 34,304) with at least one ovary at baseline. After a median of 15 years’ follow-up, ovarian cancer deaths occurred in 187 participants in the intervention arm and 176 deaths in the usual care arm, with no significant difference between the two arms. In the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) trial, 202,638 postmenopausal women were randomized to multimodality screening with annual CA 125 with triage to ultrasound ( n = 50,640), annual transvaginal ultrasound alone ( n = 50,639), or no screening ( n = 101,359). After 11 years of median follow-up, ovarian cancer was diagnosed at similar rates (~0.6%) in each group. Primary analysis did not demonstrate a difference in mortality reduction; however, there is possible evidence of mortality reduction in years 7 to 14 in the multimodality screening group.
The role of additional biomarkers, such as human epididymis protein 4 (HE4), has also been evaluated. A prospective study of 218 women with a pelvic mass completed a symptom review and evaluation of CA 125 and HE4 levels. In the 66 patients with ovarian cancer, the symptom index was abnormal in 87.9%, CA 125 in 74.2%, and HE4 in 60.6%. The sensitivity and specificity of two out of three abnormal tests was 79% and 91%, respectively. Validation studies and the evaluation of other biomarkers are ongoing.
The NIH Consensus Development Conference on ovarian cancer screening in 1994, which currently still stands, reached the following conclusions: There is no evidence available yet that the current screening modalities of CA 125 measurement and transvaginal ultrasound can be used effectively for widespread screening to reduce mortality from ovarian cancer or that their use will result in decreased rather than increased morbidity and mortality.
Clearly, it is important to identify and validate effective screening modalities. Currently available technology for screening should be used in the context of clinical trials to determine the efficacy of these modalities and their effect on ovarian cancer mortality. In addition, research must be continued to identify additional markers and imaging techniques that will be useful. If a woman were undergoing pelvic surgery, removal of the ovaries at that time would almost fully eliminate her risk of ovarian cancer (although there remains a small risk of peritoneal cancer). In a premenopausal woman who is considered at general risk, the discussion of estrogen replacement therapy is important before removal of the ovaries because the risk of premature menopause, potential for osteoporosis, and increased all-cause mortality may outweigh the risk of ovarian conservation and the potential for ovarian cancer.
Staging
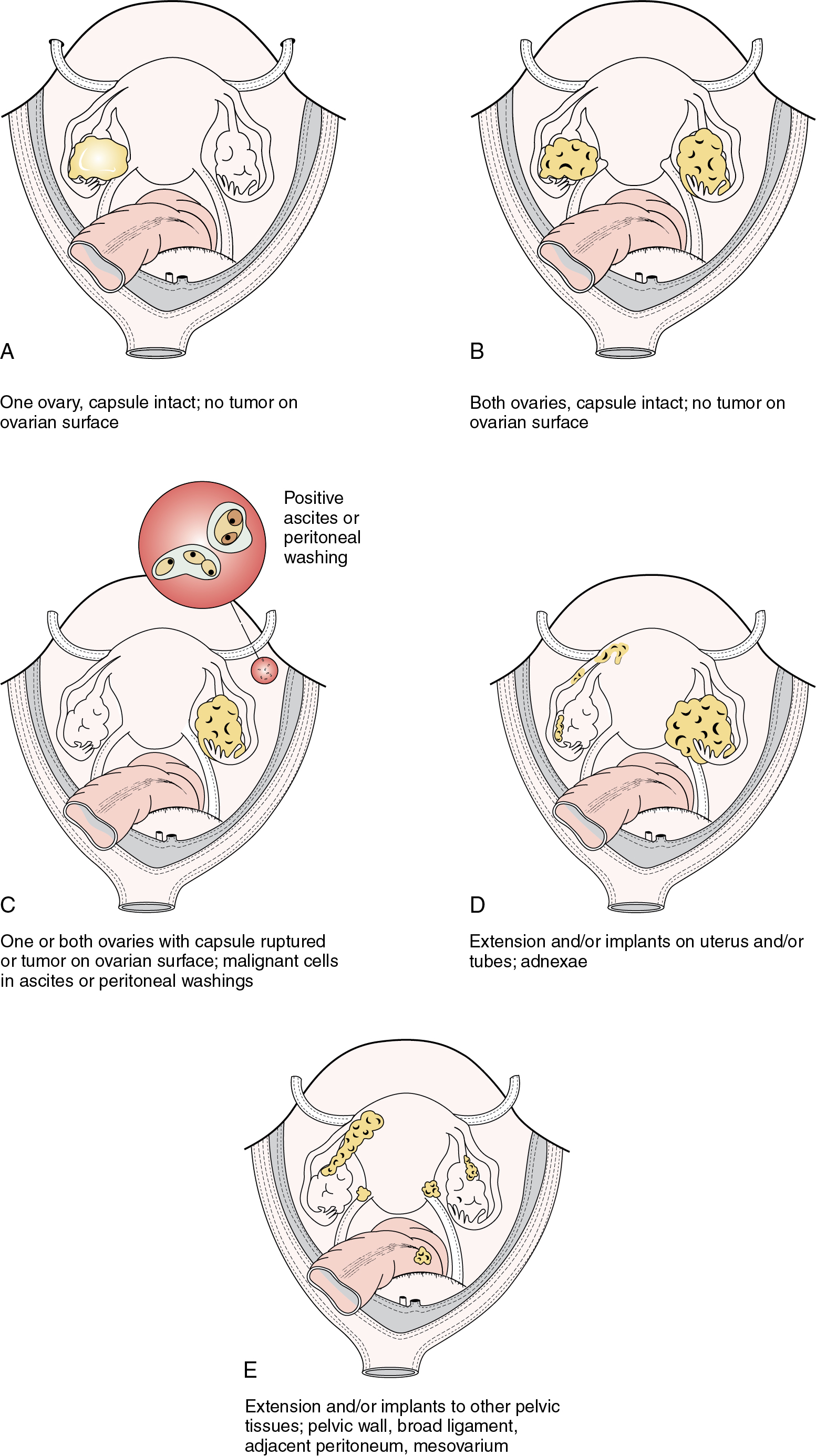
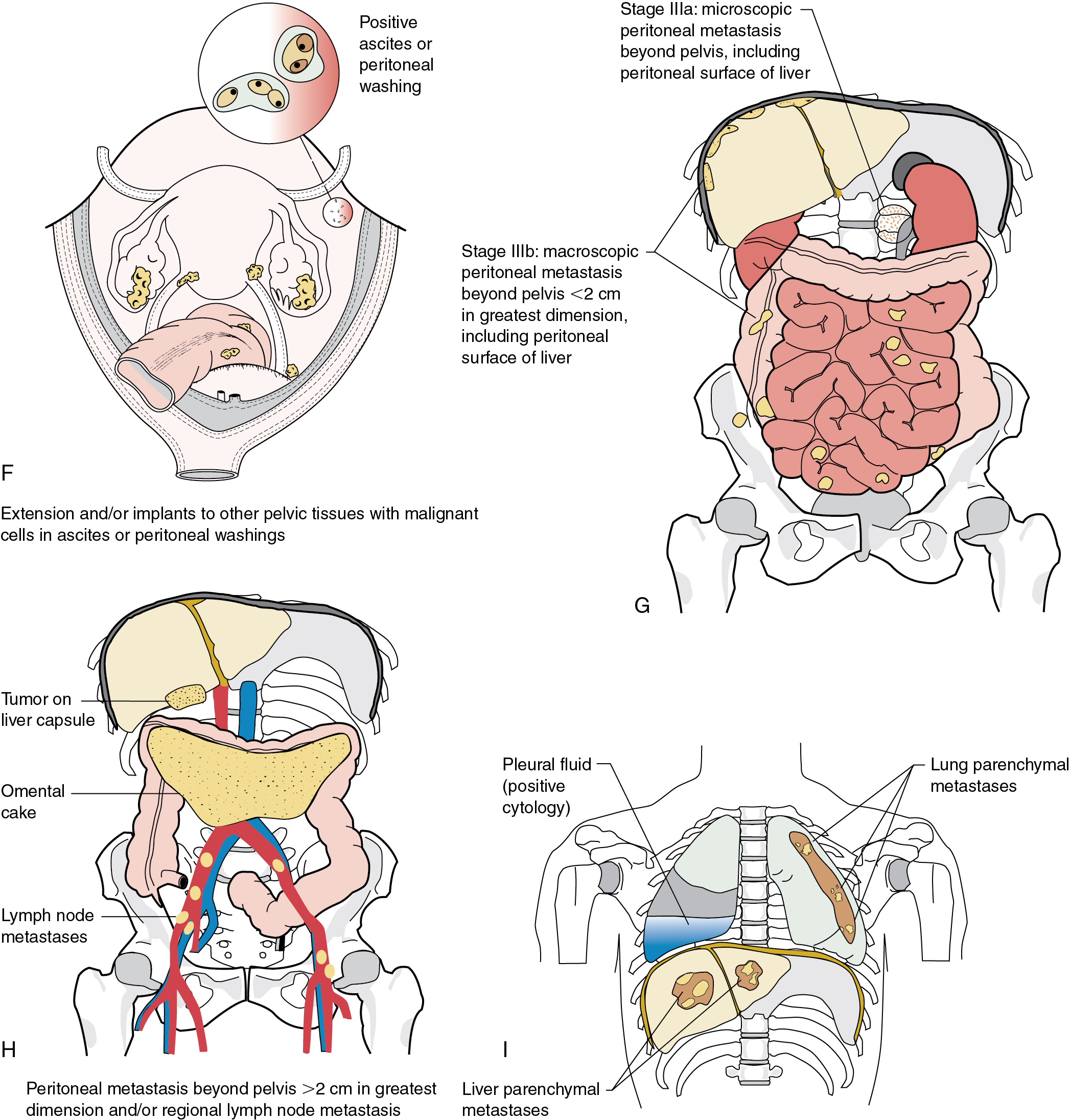
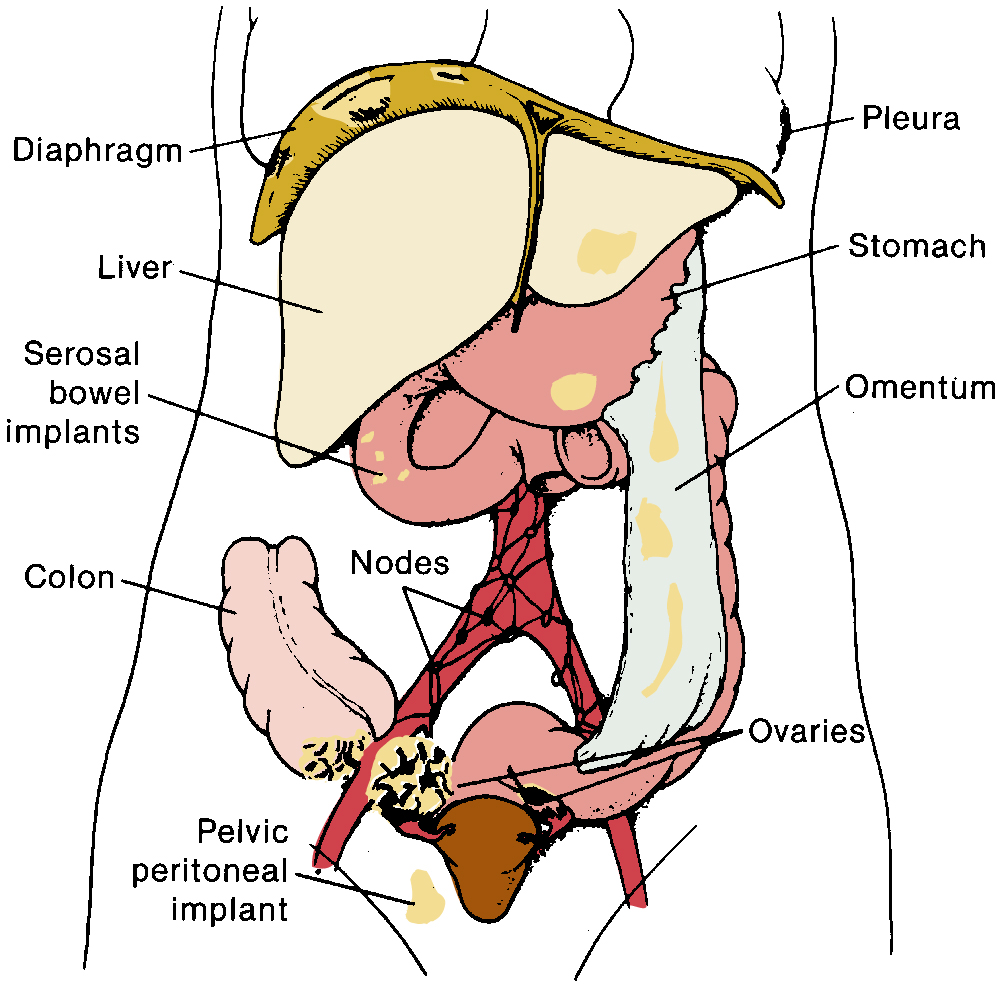
The staging of ovarian cancer is surgical ( Table 9.8 ) and based on the surgical and pathologic findings ( Fig. 9.5 ). A vertical midline incision is recommended to remove the neoplasm and to visualize the entire abdominal cavity, including the undersurface of the diaphragm. A Pfannenstiel incision is ill advised in a patient suspected of having an ovarian malignancy. Ovarian cancer classically involves the entire peritoneal cavity ( Fig. 9.6 ). Although lymphatic spread to retroperitoneal nodes is common in ovarian cancer, the disease most often spreads intraperitoneally; free-floating cells shed from the primary tumor are capable of implanting on any peritoneal surface. Any peritoneal fluid (ascites) found when the peritoneal cavity is opened should be aspirated and submitted for cytologic examination. In the absence of peritoneal fluid, washings should be taken by lavage of the peritoneal surfaces, including the undersurface of the diaphragm. Care should be taken to visualize and palpate all peritoneal surfaces, particularly the diaphragm, the surface of the liver, the lateral abdominal gutters, and the small and large bowel mesentery. The omentum should be removed because microscopic disease is often present. Recommended surgical procedures are presented in Table 9.9 . If the disease is limited to the pelvis, the primary tumor should be removed intact. All suspicious surfaces in the peritoneal cavity should be removed as biopsy specimens. This includes adhesions, which should be excised, because they often contain microscopic disease. Even when normal appearing, biopsies from the peritoneal surface may detect microscopic disease. Pelvic and aortic lymph nodes may be involved 10% to 20% of the time in apparent stage I disease, and lymphadenectomy is an important diagnostic procedure. Baiocchi and associates reviewed their experience in 242 women who had pelvic and aortic lymphadenectomy in whom cancer was apparently confined to the ovaries (stage I), and nodal metastasis was found in 32 patients (13%). Patients with serous adenocarcinoma had the highest incidence of node metastasis (25%). Those with grade III lesions had 39% metastasis compared with 6% with grade I and grade II cancers. There were 33 women with low malignant potential tumors, and 7 (21%) had nodal metastasis. When only one to three nodes were involved, metastases were usually ipsilateral, but these patients could also have metastases to the common iliac or aortic nodes. Positive aortic nodes were also found in the absence of positive pelvic nodes. Without thorough surgical staging, occult metastasis may be present and missed, leading to inadequate subsequent therapy ( Table 9.10 ). Proper staging is important for treatment planning and for providing an accurate prognosis .
| FIGO Stage | Description |
|---|---|
| I | Tumor confined to ovaries or fallopian tube(s) |
| IA | Tumor limited to one ovary (capsule intact) or fallopian tube; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings |
| IB | Tumor limited to both ovaries (capsules intact) or fallopian tubes; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings |
| IC |
|
| II | Tumor involves one or both ovaries or fallopian tubes with pelvic extension (below pelvic brim) or primary peritoneal cancer |
| IIA | Extension and/or implants on uterus and/or fallopian tubes and/or ovaries |
| IIB | Extension to other pelvic intraperitoneal tissues |
| III | Tumor involves one or both ovaries or fallopian tubes or primary peritoneal cancer with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes |
| IIIA |
|
| IIIB | Macroscopic peritoneal metastasis beyond the pelvis <2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes |
| IIIC | Macroscopic peritoneal metastasis beyond the pelvis >2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes (includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ) |
| IV |
|
|
| Localization | Frequency (%) | Range (%) |
|---|---|---|
| Peritoneal cytology | 22 | 10–46 |
| Diaphragm | 9 | 0–44 |
| Omentum | 5 | 0.7 |
| Pelvic nodes | 8 | 0–20 |
| Para-aortic nodes | 12 | 0–25 |
| Pelvic peritoneum | 9 | 6–10 |
| Abdominal peritoneum | 8 | 7–9 |
| Bowel mesentery-serosa | 6 | 3–13 |
Therapeutic options for primary treatment
The most common epithelial cancers of the ovary are histologically categorized as serous, mucinous, endometrioid, and clear cell (mesonephroid) types ( Figs. 9.7–9.10 ). Most large studies demonstrate that these different histologic types have relatively similar outcomes with existing therapy, stage for stage and grade for grade. Mucinous and endometrioid subtypes are more commonly found in earlier stages. There are ongoing studies to investigate targeted therapies in certain cell types, most notably mucinous and clear cell cancers. However, based on our current data, the prognosis, survival, and therapy for these various forms of epithelial cancer will be considered collectively.
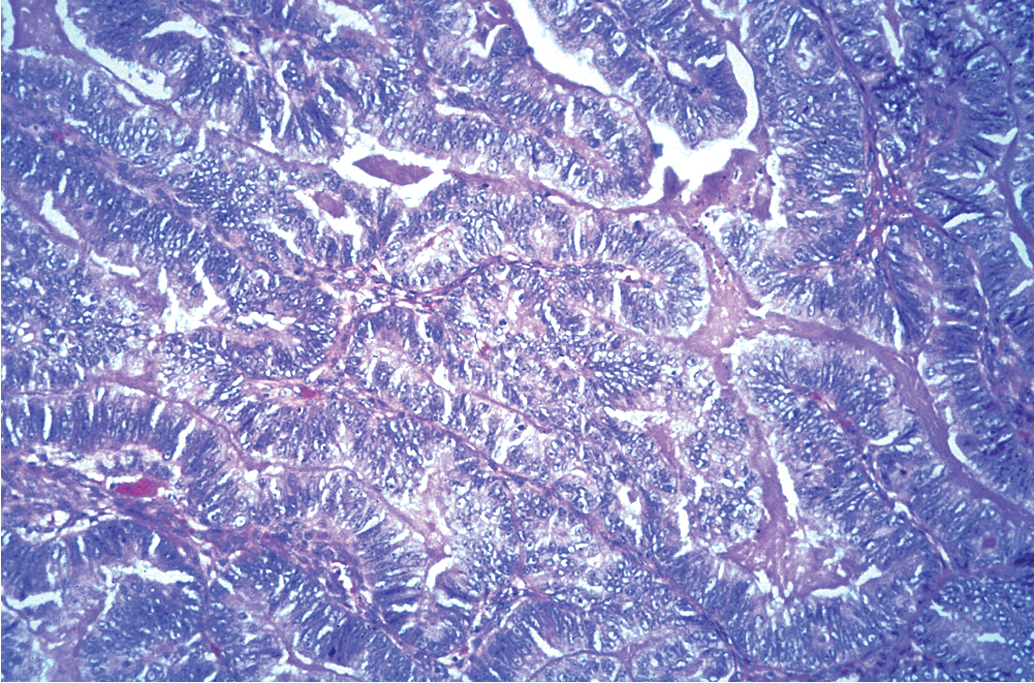
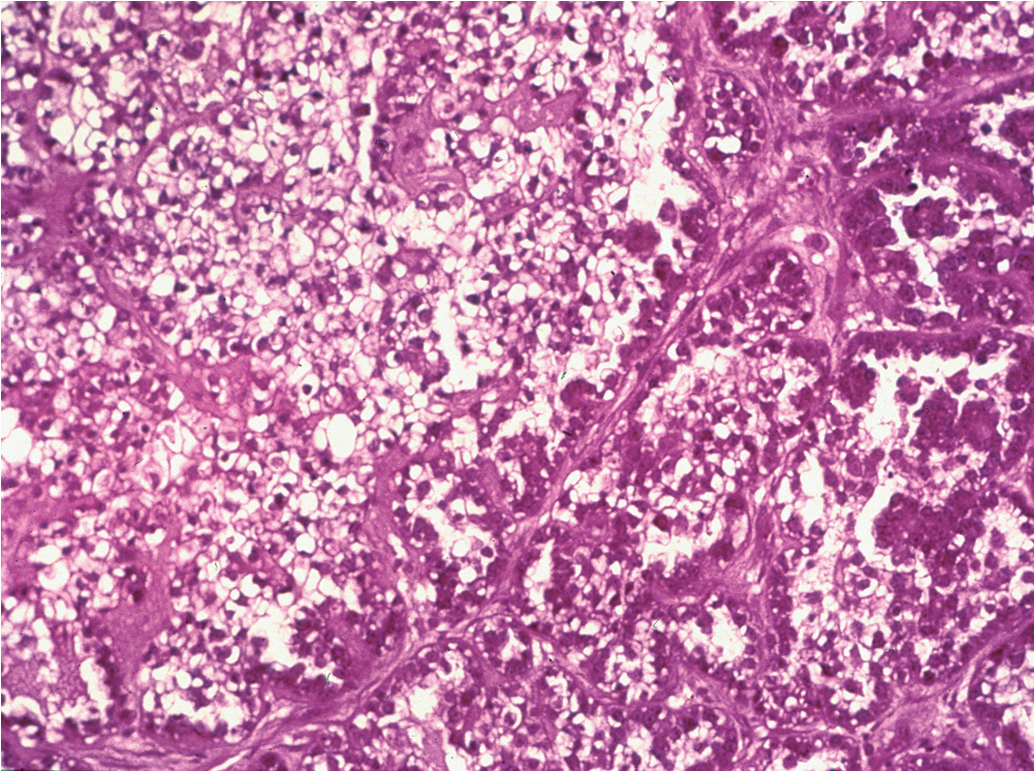
The unifocal theory of ovarian epithelial cancer growth suggests that the disease initially grows locally, invading the capsule and mesovarium, and then invades adjacent organs by local extension and lymphatic spread. Cells on the surface exfoliate into the peritoneal cavity, where they circulate and implant on free peritoneal surfaces. Local and regional lymphatic metastasis may involve the uterus, fallopian tubes, and pelvic and aortic lymph nodes.
Woodruff suggested a multifocal mechanism of disease spread, whereby the entire coelomic epithelium can give rise to this lesion after exposure to carcinogenic agents that enter the peritoneal cavity from the vagina through the fallopian tubes. Indeed, the lesion could then originate in a multifocal distribution “like a measles rash” over large portions of the coelomic epithelium. Although the early precursors leading to advanced ovarian cancer are incompletely understood, more current molecular evidence favors the unifocal origin theory.
As RRSO has become more common, a moderate body of literature suggests that a significant percentage of “ovarian cancers” may actually arise in the fallopian tube fimbria with metastasis to the ovaries. Kindelberger and colleagues have reported a potential causal relationship based on identification of tubal intraepithelial carcinomas (TICs) and analysis of p53 mutations therein.
Regardless of origin, the most important prognostic variable for each patient is the extent, or “stage,” of disease. A staging system has been devised that allows treatment results in patients with similar prognostic factors to be compared among different institutions. Survival is affected by the cancer stage ( Table 9.11 ), grade of differentiation, gross findings at surgery, amount of residual tumor after surgery, and additional treatment required.
| Stage | Incidence | 5-Year Survival (%) |
|---|---|---|
| Ia | 20%–25% | 87 |
| Ib | 71 | |
| Ic | 79 | |
| IIa | 10%–15% | 67 |
| IIb | 55 | |
| IIIa | 45%–60% | 41 |
| IIIb | 25 | |
| IIIc | 23 | |
| IV | 5%–10% | 11 |
Stages IA, IB, and IC
Careful surgical staging is critical in the management of stage I invasive ovarian cancer and should include bilateral salpingo-oophorectomy, hysterectomy, omentectomy, and pelvic and aortic lymph node sampling, with peritoneal biopsies and washings. Accurate staging forms the cornerstone of clinical decision-making regarding the type and duration of adjuvant therapy.
The use of adjuvant therapy and its role in stage I ovarian cancer have been investigated. Through three GOG studies in early-stage (I and II) ovarian cancer, use of platinum combination therapy became the preferred regimen because it demonstrated better survival outcomes and an improved, although not statistically significant, toxicity profile over intraperitoneal (IP) colloid 32P.
European investigators reported their experience with platinum-based therapy in early-stage ovarian cancer. The European gynecologic groups reported a combined analysis of the International Collaborative Ovarian Neoplasm (ICON) 1 and the Adjuvant Chemotherapy In Ovarian Neoplasm (ACTION) trials. More than 900 patients with early-stage ovarian cancer received either platinum-based adjuvant chemotherapy or observation until chemotherapy was indicated. After a median follow-up period of more than 4 years, the improved overall survival (OS) (82% chemotherapy vs. 74% observation) and recurrence-free survival (76% vs. 65%, respectively) rates at 5 years favored treatment with platinum-based therapy. Although not all patients had undergone comprehensive staging, a subgroup analysis of these patients was reported. In the European Organization for Research and Treatment of Cancer (EORTC)-ACTION trial of 448 patients, the authors noted that the benefit of chemotherapy was limited to patients who lacked comprehensive surgical staging, questioning the necessity of adjuvant therapy in the patient with well-staged early ovarian carcinoma.
The next evolution of GOG trials involved the addition of paclitaxel to a platinum-based regimen because the treatment protocols for advanced-stage disease had demonstrated improved survival by replacing cyclophosphamide with paclitaxel. GOG 157 evaluated carboplatin (area under the curve [AUC] 7.5) and paclitaxel (175 mg/m 2 ) in high-risk, early-stage disease, randomly assigning patients to three versus six chemotherapy cycles. Over a 3-year interval, 457 patients were enrolled and evaluated after a median follow-up period of 6.8 years. Although the recurrence rate was 24% lower with the six cycles ( P = .18), OS was similar for the two arms (hazard ratio [HR], 1.02).
The study concluded that the additional three cycles contributed to increased toxicity (11% grade III/IV neurotoxicity vs. 2% with only three cycles) without an OS advantage. However, an exploratory subanalysis showed that patients with high-grade serous or clear cell tumors had a decreased risk of recurrence when six cycles were administered compared with only three. Building on the hypothesis that low-dose paclitaxel may have antiangiogenic activity, GOG 175 tested whether the addition of 24 weekly paclitaxel treatments (60 mg/m 2 ) to the initial three cycles of carboplatin and paclitaxel would improve outcomes. Although the recurrence rate was 20% lower in patients receiving extended paclitaxel, the result was not statistically significant, and there was no appreciable difference in survival outcomes.
In the young woman with stage IA disease who is desirous of further childbearing, unilateral salpingo-oophorectomy may be associated with minimal increased risk of recurrence, provided a careful staging procedure is performed and due consideration is given to grade and apparent self-containment of the neoplasm. After conservative treatment for invasive ovarian cancer, term delivery rates have been reported as high as 30%, and successful pregnancy outcomes have been reported after adjuvant chemotherapy. Conservative surgery is generally not recommended for patients with clear cell, carcinosarcoma, or grade III tumors and when disease is present outside the ovaries. A careful discussion regarding the risks and benefits of this approach is essential because 12% of patients undergoing fertility-sparing ovarian cancer surgery experienced recurrence and 4% of patients died from their disease.
In the management of stage I ovarian cancer, the physician must weigh the possible benefits of adjuvant chemotherapy against the risks. It appears reasonable to not recommend adjuvant therapy for patients with stage IA, IB, grade 1 and 2 lesions who have been comprehensively staged. Patients with stage I, grade 3 and stage IC disease present a more difficult problem because the incidence of recurrence in this group approaches 50% in some series. We favor treatment with platinum-based combination chemotherapy even though no clear data show a survival advantage over single-agent therapy. Because combination therapy is our best treatment for metastatic disease, it is difficult to withhold the apparent best therapy from a group of patients who may have the best situation for a chemotherapy “cure.” In addition, this group is usually younger and better able to tolerate combination chemotherapy.
Stages IIA and IIB
In most institutions, the therapy of choice for stage IIA and stage IIB disease is staging surgery followed by platinum-based combination chemotherapy. Careful surgical staging is essential to successful treatment planning. A combined analysis of GOG 95 and 157 revealed that the stage II patients represented a disproportionate percentage of the recurrences. The GOG now includes stage II patients in their clinical trials of patients with advanced-stage disease. Outside of a clinical trial, patients with stage II disease should be managed in a similar fashion to optimally debulked stage III disease.
Stage III
In addition to surgical staging, every reasonable effort should be made to remove all visible metastatic tumors. Retrospective studies have strongly suggested that the survival rate in patients with stage III disease is related to the amount of residual tumor after surgery, such that patients with no macroscopic residual tumor appear to have the best prognosis after primary chemotherapy ( Fig. 9.11 ). The recommendation is for platinum-based chemotherapy for this group of patients because of the excellent response rates reported in the literature (further detailed later).
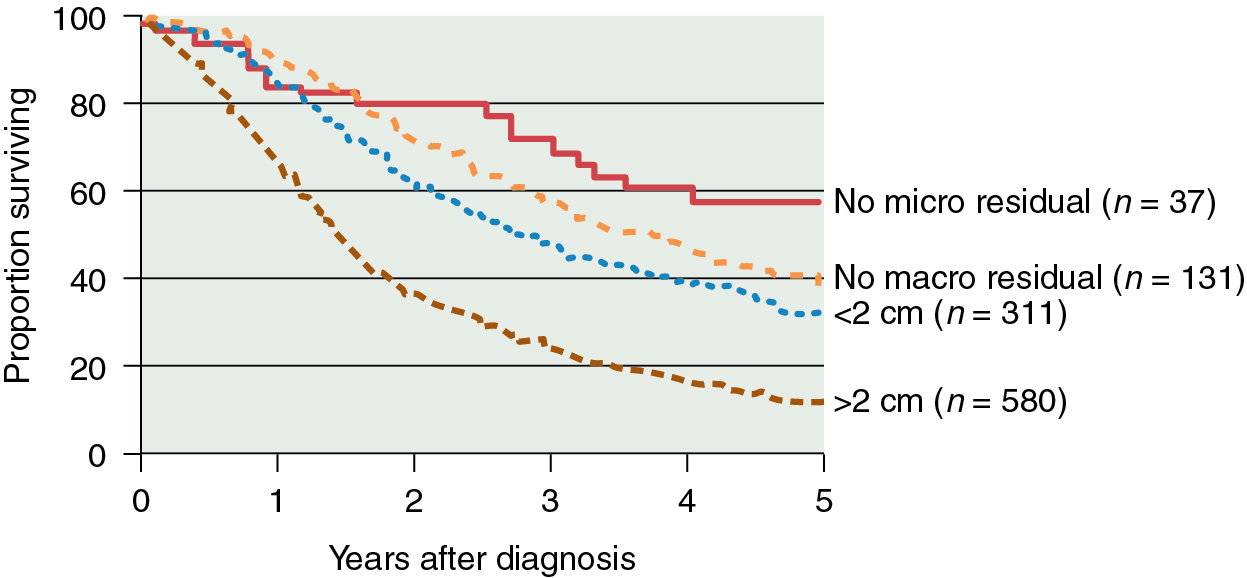
The duration of multiple-agent therapy is usually six cycles, with consideration of maintenance therapy as discussed in the following section. If after primary chemotherapy the patient has no clinical evidence of disease (clinical complete response), a second-look procedure was often considered in the past to ascertain the presence of subclinical residual disease. However, a secondary data analysis of GOG 158 suggested no apparent outcome advantage from second-look surgery, and, for the most part, second-look surgery is no longer recommended.
Stage IV
The ideal management of stage IV disease may be neoadjuvant chemotherapy followed by interval cytoreductive surgery and additional chemotherapy versus surgery to remove as much cancer as possible (to optimal/no gross residual) followed by the administration of chemotherapy. The role and benefit of surgical cytoreduction in these patients are detailed in the next section. The OS is worse for this group of patients than for those of other stages, as expected ( Fig. 9.12 ).

Surgery
Maximal surgical effort
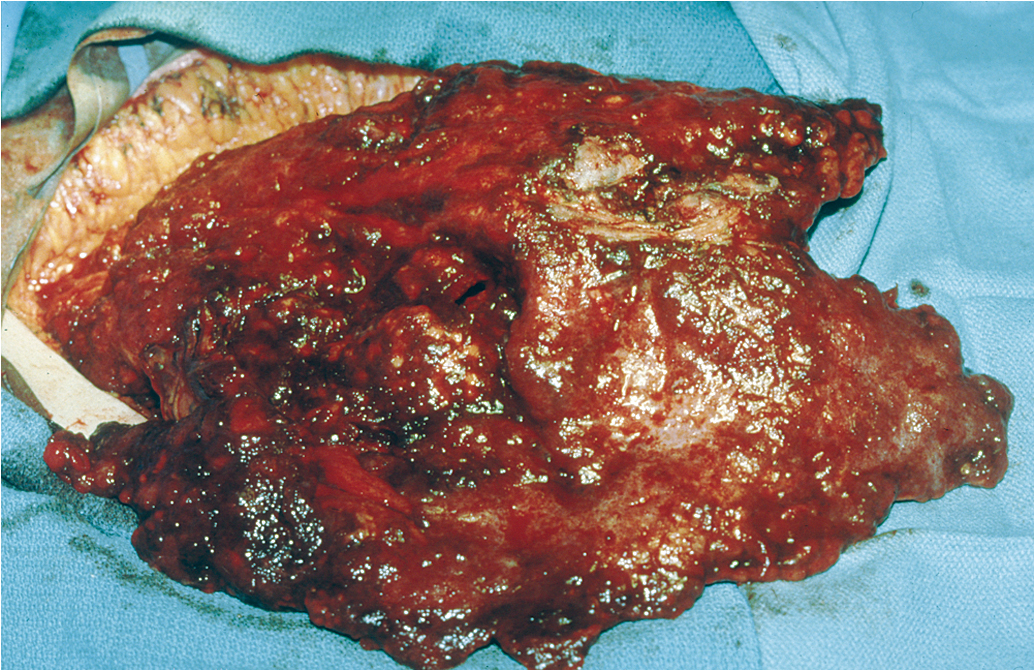
The goal of primary surgery for advanced ovarian cancer should be to remove all visible tumor and to use all reasonable surgical means to accomplish this goal ( Fig. 9.13 ). In the 1970s, Griffiths and coworkers evaluated the importance of postsurgical tumor residual by using a multiple linear regression equation with survival as the dependent variable to control simultaneously for the multiple therapeutic and biologic factors that contribute to the ultimate outcome in the individual patient. The most important factors proved to be histologic grade of the tumor and size of the largest residual mass after primary surgery. In the original study by Griffiths et al., the operation itself contributed nothing to survival unless it effected reduction in the size of the largest residual tumor mass to less than the limit of 1.6 cm.
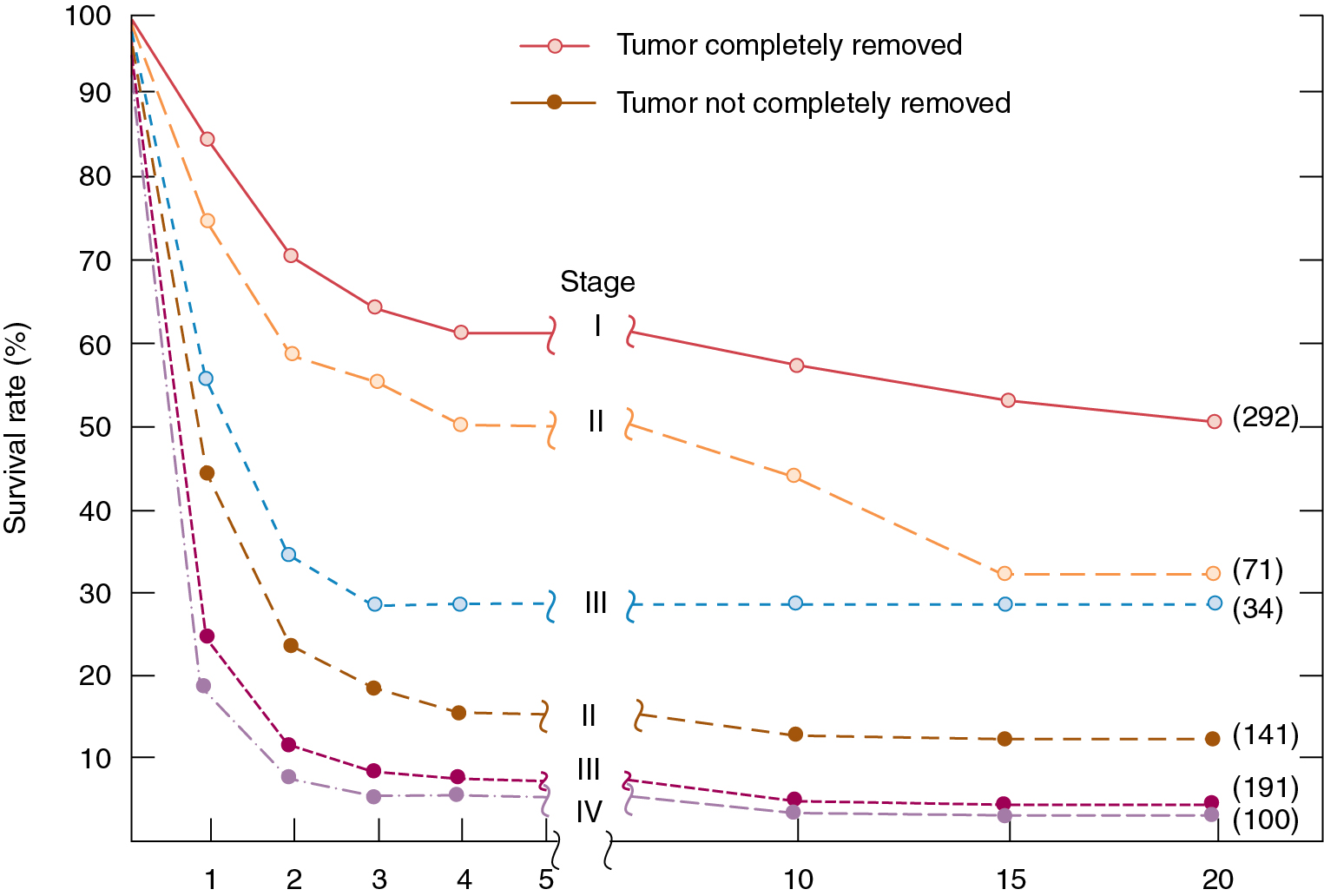
Cytoreductive or “debulking” procedures have gained considerable attention in the management of ovarian cancer. The concept is to reduce the residual tumor burden to a level at which subsequent chemotherapy can be most effective. A careful dissection by a well-trained gynecologic oncologist can often remove disease that on first impression appears to be unresectable. Removal of large ovarian masses and omental involvement may reduce the tumor burden by 80% to 99% ( Fig. 9.14 ) . The theoretical value of debulking procedures lies in reducing the number of tumor cells and the advantage this affords to adjuvant therapy. This is especially relevant in bulky chemosensitive solid tumors such as ovarian cancer, in which removal of large numbers of cells in the resting phase (G0) may propel the residual cells into the more vulnerable proliferating phases. Several careful retrospective studies have repeatedly demonstrated improved survival rates in patients who can be surgically reduced to minimal tumor burden. The GOG has attempted to better define primary cytoreductive surgery with a detailed analysis of the results of surgery in patients with advanced disease. Their initial study compared survival of the patients with stage III disease who were found at surgery to have abdominal disease of 1 cm or smaller with that of patients found to have disease larger than 1 cm but whose tumors were surgically cytoreduced to 1 cm or less. If surgery was the only important factor, survival should have been equivalent in both groups; however, this was not the case. Patients found to have small-volume disease at baseline survived longer than patients who had cytoreduction to small-volume disease at surgery, suggesting that the tumor biology also carries prognostic significance. In a second study, GOG investigators evaluated the effect of the diameter of the largest residual disease on survival in patients with suboptimal cytoreduction. They demonstrated that cytoreduction so that the largest residual mass was 2 cm or less resulted in a significant survival benefit, but all residual diameters larger than 2 cm had equivalent survival. Therefore, unless the mass could be cytoreduced to 2 cm or less, residual diameter did not influence survival. In evaluating optimal and suboptimal cytoreduction, these GOG investigators showed that three distinct groups emerged: microscopic (no visible) residual, residual disease of less than 2 cm, and residual disease of greater than 2 cm ( Fig. 9.15 ). It is clear from these studies that, although patients with microscopic disease had a 4-year survival rate of approximately 60%, patients with gross disease 2 cm or less had a 4-year survival rate of 35%. However, patients whose disease could not be cytoreduced to 2 cm or less had a 4-year survival rate of less than 20%. Most striking was the failure of cytoreductive surgery to improve survival unless the largest diameter of residual disease was 2 cm or less. Therefore maximal cytoreductive surgery that results in microscopic residual disease results in the best outcome .
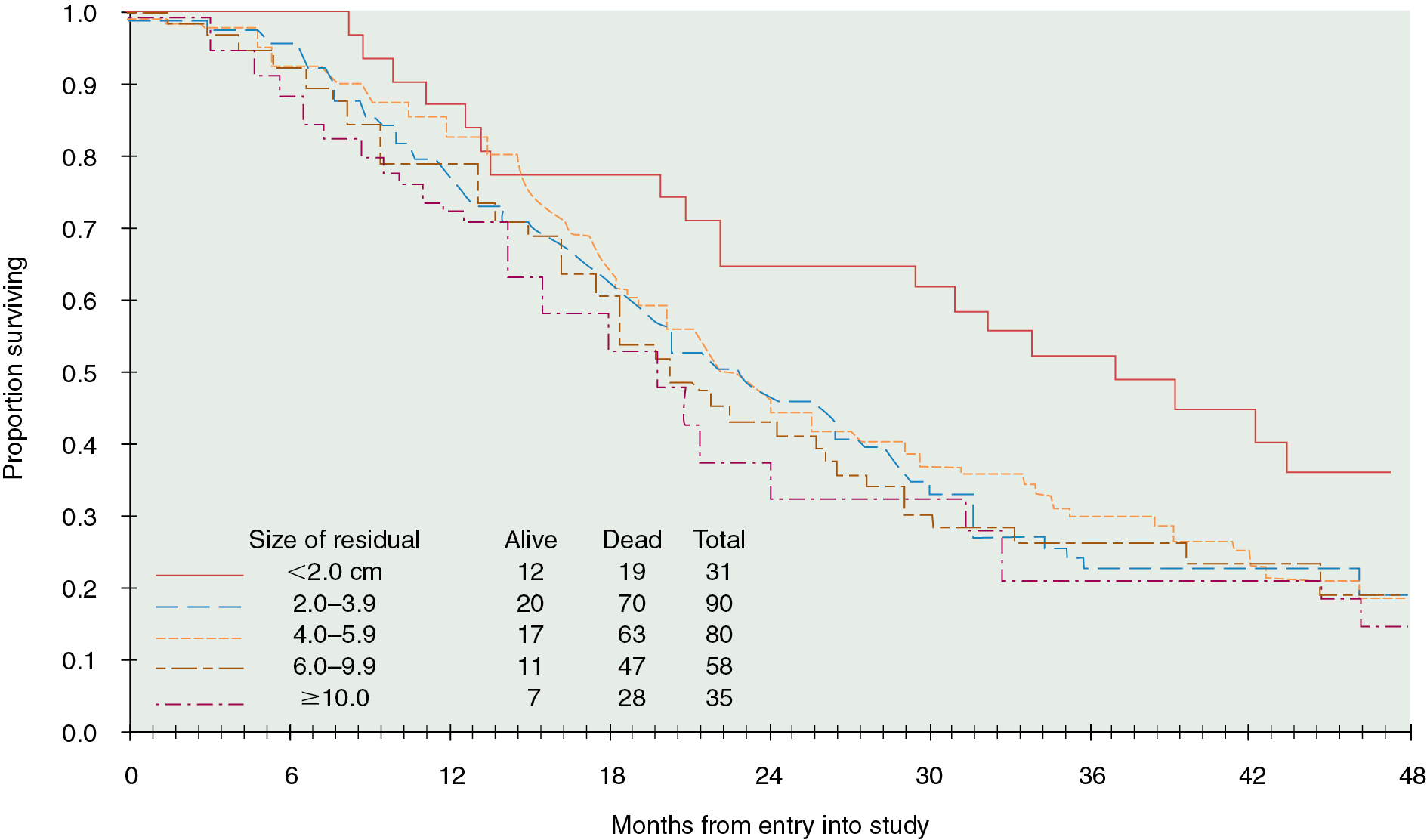
An inverse correlation between the amount of residual disease after surgery and OS has been consistently associated. In 2002, Bristow and associates published a meta-analysis of published studies at the time, in which the impact of cytoreduction was clearly demonstrated. By comparing currently available studies of advanced ovarian cancer patients undergoing surgical cytoreduction, they demonstrated that for each 10% increase in the proportion of patients undergoing optimal cytoreduction, the median survival rate increased by 5.5% to 6%. Although the current accepted definition of “optimal cytoreduction” is no visible residual tumor larger than 1 cm in greatest dimension, the best reported outcomes occur in patients who have complete cytoreduction or no visible residual disease. For example, retrospective analysis of three large randomized controlled trials by the Arbeitsgemeinschaft GynaKologische Onckologie (AGO) and GINECO revealed that progression-free survival (PFS) was significantly improved in patients who had a complete resection. Importantly, there was no difference in survival between patients who had residual disease of 1 to 10 mm and those who had greater than 10 mm residual disease.
In an effort to remove visible tumor deposits and achieve optimal cytoreduction, gynecologic oncologists frequently perform radical surgical procedures beyond those required for standard staging. Multiple studies have also reported that large or small bowel resection, which may be necessary in up to 50% of cases to achieve optimal cytoreduction, can be performed safely and has been associated with a subsequent improvement in survival. Upper abdominal cytoreduction has also been shown to be safe and effective but may require additional training or assistance. Cliby and colleagues reported procedures such as liver resection, splenectomy, and diaphragm resection to be possible and associated with an acceptable morbidity. Chi and coinvestigators demonstrated that the addition of upper abdominal procedures to achieve optimal cytoreduction resulted in a median survival time longer than 5 years, which was comparable with that of patients who did not require these extensive procedures for optimal cytoreduction. Improvements in surgical cytoreduction have developed in consort with additional advances in intensive care and postoperative care including enhanced recovery, allowing additional benefits for our ovarian cancer patients.
The role of cytoreductive surgery in stage IV cancers has been investigated. Several studies have suggested that optimal debulking can be performed in many of these patients with beneficial effect. A study by Chi and colleagues identified 92 patients; optimal debulking was achieved in 45%, with a median survival time of 40 months compared with 18 months for suboptimal debulking. An ancillary study by the GOG demonstrated that the ability to achieve cytoreduction to less than 5 cm residual disease in stage IV patients rendered some survival advantage, although the most favorable subgroup was patients reduced to no visible residual disease. Aletti and colleagues also demonstrated that using radical procedures to perform cytoreduction to no visible residual disease resulted in median survival times longer than 3 years in stage IV patients. It currently seems appropriate that patients with a diagnosis of advanced ovarian cancer should undergo resection of all masses when technically feasible. For patients known or suspected to have thoracic metastases, the use of video-assisted thoracic surgery (VATS) can be diagnostic, potentially therapeutic, or both. Positive findings on VATS may allow for intrathoracic cytoreduction in select cases or may indicate which patients are best served with neoadjuvant chemotherapy as a result of unresectable pleural disease.
Even though cytoreductive surgery consistently appears to have therapeutic value, controversy continues. The primary unresolved issue is whether the poor prognosis with bulky disease is caused by the presence of increased tumor burden (in which case cytoreductive surgery is of potential benefit) or whether it is associated with differences in tumor biology or a decreased sensitivity to chemotherapeutic regimens. If these latter possibilities are indeed the case, cytoreductive surgery is not likely to have a major impact on survival. The implication is that patients who have disease that can be cytoreduced are a select group with good prognoses on the basis of factors independent of the surgery. Furthermore, if chemotherapy is delayed because of complications of surgery, this may have a deleterious effect on long-term survival. The percentage of patients with advanced ovarian cancer who can effectively undergo primary cytoreductive surgery seems to range from 43% to 87%, depending on the investigators reporting. This difference may not reflect only the individual skills of the surgeons but also may be influenced by different referral patterns and other selection factors. Neoadjuvant chemotherapy has also been investigated as a means by which to address this issue; these studies are described in the subsequent section.
The role of lymphadenectomy in patients with advanced disease has been debated. Lymph node involvement is common in patients with advanced disease (>50%); however, the question of whether lymphadenectomy improves survival was unknown. It has been suggested by some that metastases in lymph nodes do not respond well to chemotherapy, and therefore these potential metastatic sites should be removed. Opponents argued that recurrence most frequently occurs in the peritoneal cavity, and therefore lymph node status has little impact on the overall disease course. Several older studies reached somewhat different conclusions. Parazzini and associates evaluated 456 women with stage III or IV disease entered in a prospective randomized chemotherapy trial. There were 161 patients with positive nodes. They noted grade 3 tumors to have a greater number with positive nodes compared with grade I or grade II tumors. This was also true of stage IV compared with stage III cancer. They did not find a difference in survival between those with positive nodes and those with negative nodes. Scarabelli and colleagues evaluated lymph node status in 98 patients with stage IIIC to IV disease who had no gross residual disease after surgery compared with 44 patients who did not undergo lymphadenectomy. Survival was significantly improved if lymphadenectomy was done. In 2005, Panici and coinvestigators reported their prospective multicenter analysis of 427 advanced ovarian cancer patients randomly assigned to lymphadenectomy or removal of bulky nodes only. Although PFS was improved in patients undergoing lymphadenectomy, OS was not different. More recently, however, the Lymphadenectomy in Patients with Advanced Ovarian Neoplasms (LION) trial was published, which evaluated 647 women with stages IIB-IV ovarian cancer and normal-appearing lymph nodes. Women with complete cytoreduction were randomized intraoperatively to receive systematic lymphadenectomy or not. Harter and colleagues found that there were no differences in PFS or OS but that there were increased rates of serious postoperative complications in the group who received lymphadenectomy. Therefore it is a commonly accepted practice to remove lymph nodes only if they are abnormal on palpation or imaging and no longer perform systematic pelvic and aortic lymphadenectomy in the setting of stage III or IV ovarian cancer that has been optimally cytoreduced to no gross residual disease.
Because a suboptimal surgical effort confers no survival benefit and adds only postoperative morbidity, accurate methods to determine which patients could be successfully resected would be useful. Both preoperative imaging and initial laparoscopy have been investigated as methods to determine the likelihood of success. Most imaging series have used preoperative CT scan to assess factors that could predict an optimal cytoreduction. Although sets of radiographic risk factors have been described, these have had poor predictive value because the majority of patients (62% to 86%) identified as having “poor prognostic indicators” have been able to undergo optimal cytoreduction. Similarly, laparoscopy in advanced ovarian cancer has limitations in terms of how complete an assessment of the peritoneal cavity is possible, and metastases at port sites have been reported. Wang and associates found that a metastasis to a port site was more common if ascites and IP carcinomatosis were present. However, the clinical significance of port site metastases is unclear because they often clinically resolve with systemic therapy. Multiple groups have proposed scoring systems for laparoscopic assessments to predict the likelihood of achieving complete cytoreduction based on operative findings, the most prominent of which was developed by Fagotti and colleagues ( Table 9.12 ). This scoring system has been prospectively validated, and there are no data to date that suggest that survival outcomes are worse if a laparoscopic assessment is performed prior to primary treatment.