Key points
- 1.
Vaccination for human papilloma virus (HPV) should decrease the incidence of high-grade squamous intraepithelial lesions (HSILs) of the vulva and vagina.
- 2.
Lichen sclerosus is associated with vulvar cancer.
- 3.
If there is any concern for malignancy, wide local excision is the treatment of choice for vulvar HSIL.
- 4.
Topical treatments such as imiquimod and cidofovir offer non-surgical management options for HSIL.
Embryology and diethylstilbestrol exposure
At approximately 12 to 14 weeks of gestation, the simple columnar epithelium that lines the vaginal portion of the uterovaginal canal begins to undergo transformation into stratified müllerian epithelium. This transformation proceeds cranially until it reaches the columnar epithelium of the future endocervical canal. The vagina, which is lined initially by simple columnar epithelium of müllerian origin, acquires stratified müllerian epithelium. The vaginal plate advances in a caudocranial direction, obliterating the existing vaginal lumen. By caudal cavitation of the vaginal plate, a new lumen is formed, and the stratified müllerian epithelium is replaced by a stratified squamous epithelium, probably from a urogenital sinus origin. Local proliferation of the vaginal plate in the region of the cervicovaginal junction produces the circumferential enlargement of the vagina known as the vaginal fornices, which surround the vaginal part of the cervix.
The administration of diethylstilbestrol (DES) through the 18th week of gestation can apparently result in the disruption of the transformation of columnar epithelium of müllerian origin to the stratified squamous successor ( Fig. 2.1 ). This retention of müllerian epithelium gives rise to adenosis. Adenosis may exist in many forms: glandular cells in place of the normal squamous lining of the vagina, glandular cells hidden beneath an intact squamous lining, or mixed squamous metaplasia when new squamous cells attempt to replace glandular cells.
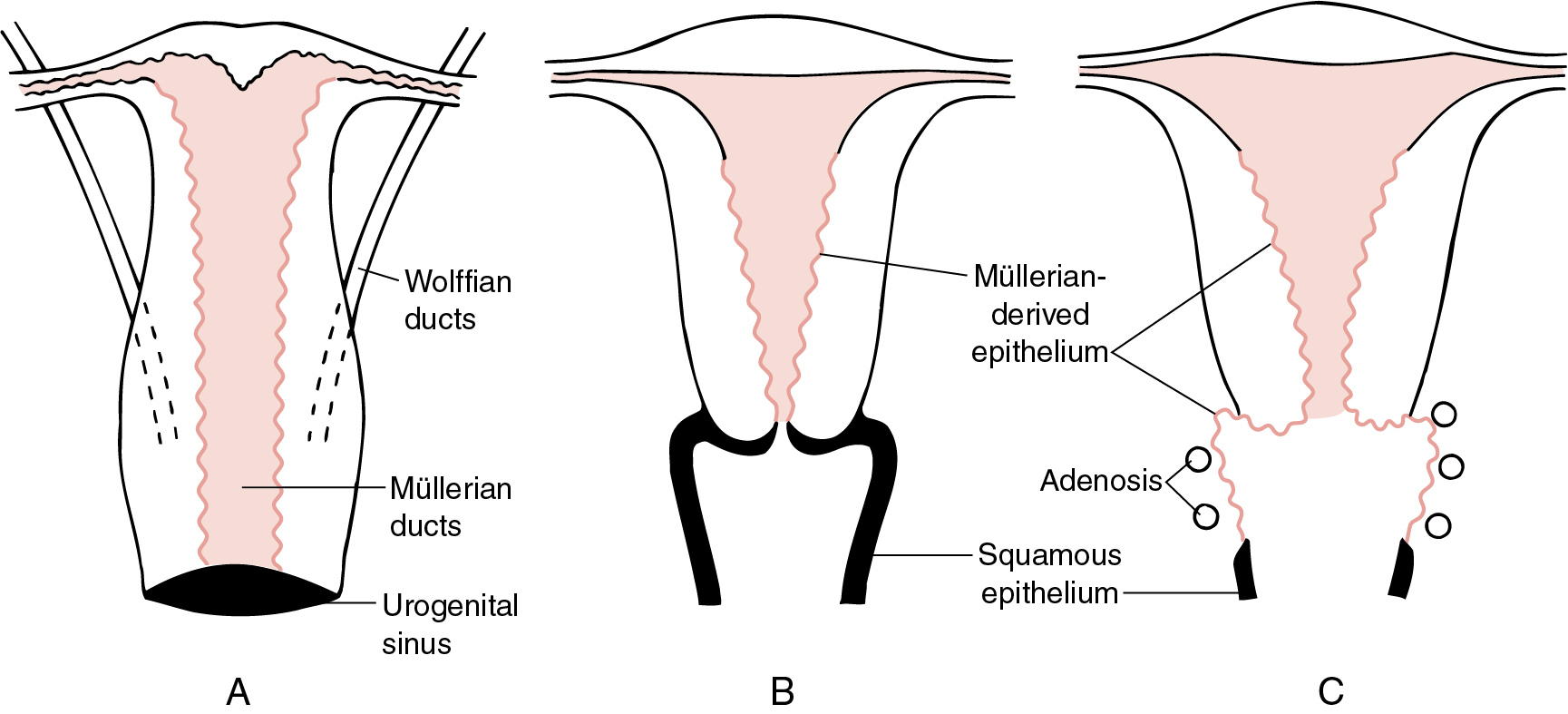
Vaginal adenosis is rarely observed in patients without a history of DES exposure and is usually clinically insignificant. The critical time of DES exposure in utero appears to be prior to 18 weeks as adenosis is more common in patients whose mothers began DES treatment early in pregnancy and is not observed if DES administration began after that point. At least 20% of women exposed to DES show an anatomic deformity of the upper vagina and cervix; transverse vaginal and cervical ridges, cervical collars, vaginal hoods, and cockscomb cervices have all been described. The transverse ridges and anatomic deformities found in one-fifth of women exposed to DES make it difficult to ascertain the boundaries of the vagina and cervix. The cervical eversion causes the cervix grossly to have a red appearance. This coloration is caused by the numerous normal-appearing blood vessels in the submucosa. With a colposcope and application of acetic acid solution, numerous papillae (“grapes”) of columnar epithelium are observed, similar to those seen in the native columnar epithelium of the endocervix. The hood ( Fig. 2.2 ) is a fold of mucous membrane surrounding the portio of the cervix; it often disappears if the portio is pulled down with a tenaculum or is displaced by the speculum. The cockscomb is an atypical, peaked appearance of the anterior lip of the cervix, and vaginal ridges are protruding circumferential bands in the upper vagina that may hide the cervix. A pseudopolyp formation (see Fig. 2.2 ) has been described that occurs when the portio of the cervix is small and protrudes through a wide cervical hood.
The occurrence of vaginal adenosis among young women without in utero DES exposure implies that an event in embryonic development is responsible. The development of the müllerian system depends on and follows formation of the wolffian, or mesonephric, system. The emergence of the müllerian system as the dominant structure appears unaffected by intrauterine exposure to DES when studied in most animal systems. However, it is apparent that steroidal and nonsteroidal estrogens, when administered during the proper stage of vaginal embryogenesis in mice, can permanently prevent the transformation of müllerian epithelium into the adult type of vaginal epithelium, thus creating a situation like adenosis. The colposcopic and histologic features of vaginal adenosis strongly support the concept of persistent, untransformed müllerian columnar epithelia in the vagina as the explanation of adenosis.
Treatment of women exposed to diethylstilbestrol
Essentially no DES has been prescribed to pregnant women since 1971, when the US Food and Drug Administration issued an alert regarding the risk of vaginal clear cell adenocarcinoma for females exposed in utero. The youngest women with in utero exposure were born in 1972, and many previously important clinical topics, such as teenage cancers, diagnosis of congenital anomalies in young women, and associated infertility and pregnancy risks, have become less relevant in everyday practice. However, an understanding of the disease process through the lifespan remains essential in order to care for this cohort of women as they age. Additionally, the evolution and understanding of DES is of great historical importance with many implications and lessons for today’s practice.
Approximately 60% of women with in utero DES exposure have vaginal adenosis, cervical-like epithelium in the vagina, which appears red and granular. This is a benign condition that does not require treatment unless symptomatic, and it will generally resolve on its own over time. The premenopausal risk of clear cell adenocarcinoma is approximately 40 times higher among women with in utero DES exposure compared with those without the exposure. The 20-year overall survival with vaginal clear cell carcinoma is approximately 70%. In unexposed women, clear cell adenocarcinoma occurs almost exclusively in menopause, while in a registry of women with in utero DES exposure, the median age of diagnosis was 22 and 80% of cases were diagnosed between the age of 15 and 30. The oldest reported patient with DES-associated clear cell adenocarcinoma was diagnosed at 51 years. Overall, the absolute number of women diagnosed and incidence of clear cell adenocarcinoma is low in women with in utero exposure (≈1.5 cases/1000 women), but the risk is significantly higher than among unexposed women. The etiology of clear cell adenocarcinoma is unclear, and evolution of adenosis to cancer has never been directly proven, only suspected. Because of the increased risk of clear cell adenocarcinoma of the cervix and vagina and an increased risk of cervical high-grade squamous intraepithelial lesion (HSIL), annual examinations with cytology testing are recommended for the entire lifetime of women with in utero DES exposure. No increased association has been observed with squamous cell cervical cancer; however, the risk of cervical intraepithelial neoplasia (CIN) II or greater was approximately doubled and the overall incidence approximately 5% in a large cohort study of women ( n = 4120) with in utero DES exposure. Similarly, the same study found the risk of breast cancer after age 40 was also nearly doubled for women with DES exposure. More recently, DES exposure has been associated with coronary artery disease and myocardial infarction (but not stroke), independent of traditional cardiovascular risk factors. Future research is also incorporating the study of third-generation “DES granddaughters,” as these women may have an increased risk of amenorrhea, irregular menstrual cycles, and preterm delivery.
All DES-exposed females should have an annual gynecologic examination with cytology screening of the cervix and vagina. If suspicious lesions are present, colposcopy and directed biopsies should be performed regardless of the cytology results. If delineations of the vagina, cervix, and endocervix are difficult, Lugol’s solution may be helpful in detecting abnormal areas. Colposcopic examination of these patients is hindered by the abnormal patterns seen with squamous metaplasia ( Fig. 2.3 ) , which can be confused with neoplastic lesions. Histologic confirmation is essential before any treatment is undertaken. Marked mosaic ( Fig. 2.4 ), and punctation patterns that normally herald intraepithelial neoplasia are commonly seen in the vagina of DES-exposed women as a result of widespread metaplasia. The purpose of regular examination is to permit detection of adenocarcinoma and squamous neoplasia during the earliest stages of development. Although many therapies have been attempted, no recommended treatment plan for vaginal adenosis exists. In most cases, the area of adenosis is physiologically transformed into squamous epithelium during varying periods of observation, and no therapy is necessary.
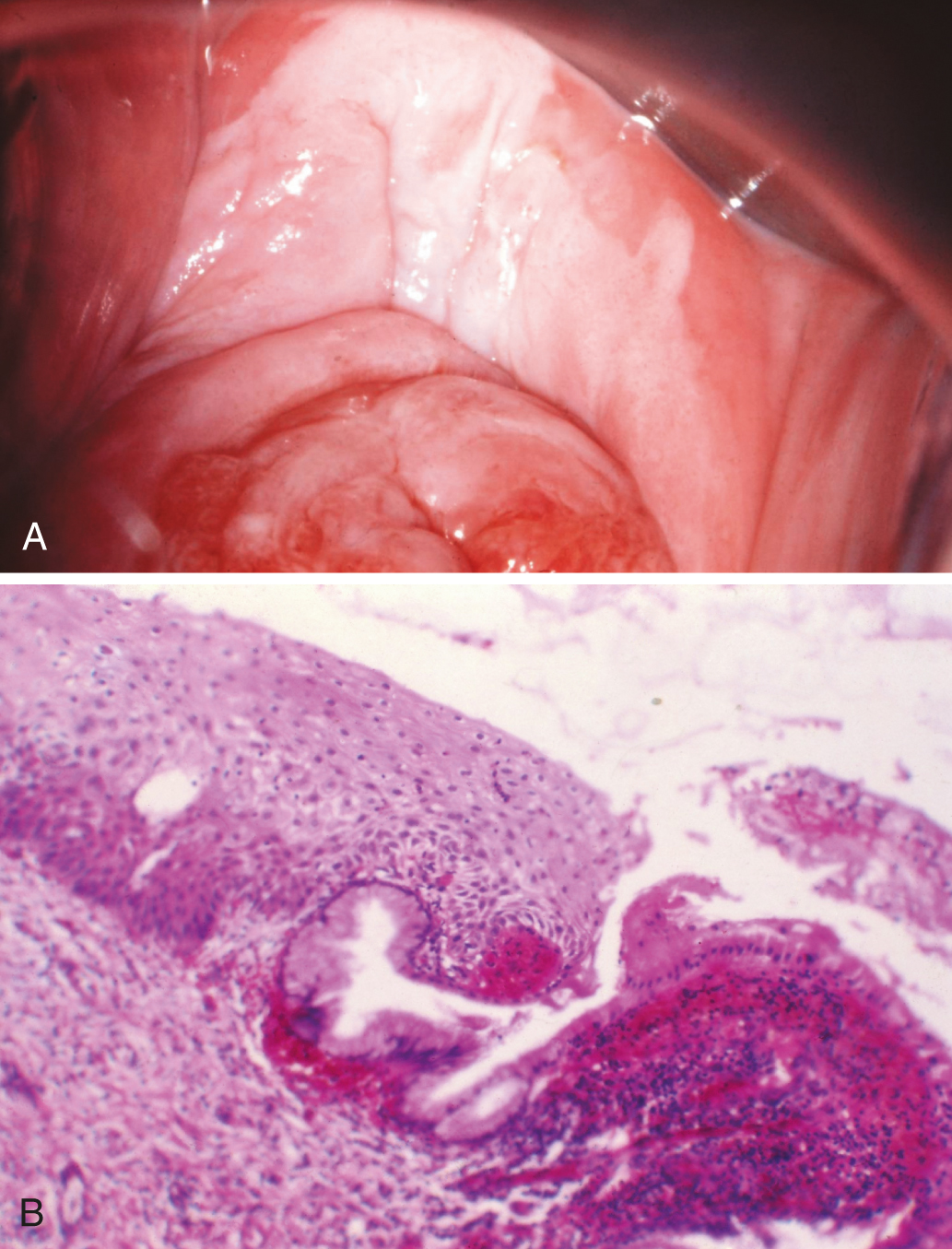

Nonneoplastic epithelial disorders of the vulva and vagina
Nonneoplastic epithelial disorders of the vulvar skin and mucosa are frequently seen in clinical practice. Diagnosis is difficult without a biopsy to provide a histologic result, and multiple changes in terminology over the past 25 years add confusion to clinical management. The current classification guideline published in 2007 by the International Society for the Study of Vulvovaginal Disease (ISSVD) ( Table 2.1 ) was updated in 2011 to expand upon the clinical characteristics of the diagnoses. The lichenoid pattern subset includes two chronic diseases, lichen sclerosus and lichen planus, which are relevant to this review because of the association of those two diseases with vulvar cancer. Lichen simplex chronicus is not included in the lichenoid subset; it is classified in the acanthotic pattern subset. This is appropriate from an oncology viewpoint because lichen simplex chronicus is not associated with invasive cancer. With similar presenting symptoms and a hyperkeratotic appearance, however, lichen simplex chronicus can be difficult to discriminate from lichen sclerosus and lichen planus on examination ( Table 2.2 ).
| Pathologic Subset | Clinical Correlate |
|---|---|
|
|
| |
| |
|
|
| |
| |
| |
|
|
| |
|
|
|
|
| |
|
|
| |
| |
|
|
| |
|
|
| |
|
| Disorder | Lesion | Genital | Other Locations |
|---|---|---|---|
| Seborrheic dermatitis | Erythema with mild scale oval plaques | Mild scaling; also “inverse type” | Central face, neck, scalp, chest, back |
| Psoriasis | Annular scaly plaques that bleed easily | Red plaques with gray-white scale | Scalp, elbows, knees, sacrum |
| Tinea | Annular plaques with central clearing | Common | Skinfolds or single “ringworm” lesion |
| Lichen simplex chronicus | Lichenified plaques, some dermatitic | Scrotum or labia majora | Nape of the neck, ankle, forearm, antecubital and popliteal fossae |
| Lichen planus | Flat-topped lilac papules and plaques | White network, erosive vaginitis | Volar wrists, shins, buccal mucosa |
Lichen simplex chronicus
Lichen simplex chronicus presents in girls and women of all ages, but it is more common in the reproductive and postmenopausal years. Lichen simplex chronicus is a chronic eczematous condition, and most women with this condition also have a history of atopic dermatitis. Pruritus is the most common symptom, and over time, scratching leads to epithelial thickening and hyperkeratosis. The status of the skin usually relates to the amount of scratching. Exacerbating agents may include heat, moisture, sanitary or incontinence pads, and topical agents. Lichen simplex chronicus may be seen in conjunction with other processes such as Candida infections or lichen sclerosus; these primary diseases must be treated in order to treat the pruritus leading to scratching and hyperkeratosis.
The locations most often involved on the vulva include the labia majora, interlabial folds, outer aspects of the labia minor, and clitoris. It is particularly common among women with chronic incontinence to find involvement of the labia majora at point of contact with incontinence pads and sparing of the medial labia and mucosa centrally where the pad is not in contact with the epithelium. Changes can also extend to the lateral surfaces of the labia majora or beyond. Areas of lichen simplex chronicus are often localized, elevated, and well-delineated, but they may be extensive and poorly defined. The appearance of lesions may vary greatly even in the same patient. The vulva often appears dusky red when the degree of hyperkeratosis is slight and may appear thickened and leathery when hyperkeratosis persists. At other times, well-defined white patches may be seen, or a combination of red and white areas may be observed in different locations. Thickening, fissures, and excoriations require careful evaluation because carcinoma may be exhibited by these same features. For this reason, biopsy is essential if there is any ambiguity in order to ascertain the correct diagnosis.
Biopsy reveals a variable increase in the thickness of the horny layer (hyperkeratosis) and irregular thickening of the malpighian layer (acanthosis). This latter process produces a thickened epithelium and lengthening and distortion of the rete pegs. Parakeratosis may also be present. The granular layer of the epithelium is usually prominent. An inflammatory reaction is often present within the dermis with varying numbers of lymphocytes and plasma cells.
Lichen sclerosus
Lichen sclerosus represents a specific disease found in genital and nongenital sites. The vulva is the most common lesion site in women. The age distribution of the disease is bimodal with peaks during the premenarchal and postmenopausal years, but the highest incidence is among postmenopausal women. Although the overall incidence and prevalence are unknown, Leibovitz and colleagues reported the prevalence among elderly women in a nursing home setting as 1 in 30. The mean age in this population was 82 years, 88% were wheelchair users, and 86% were incontinent.
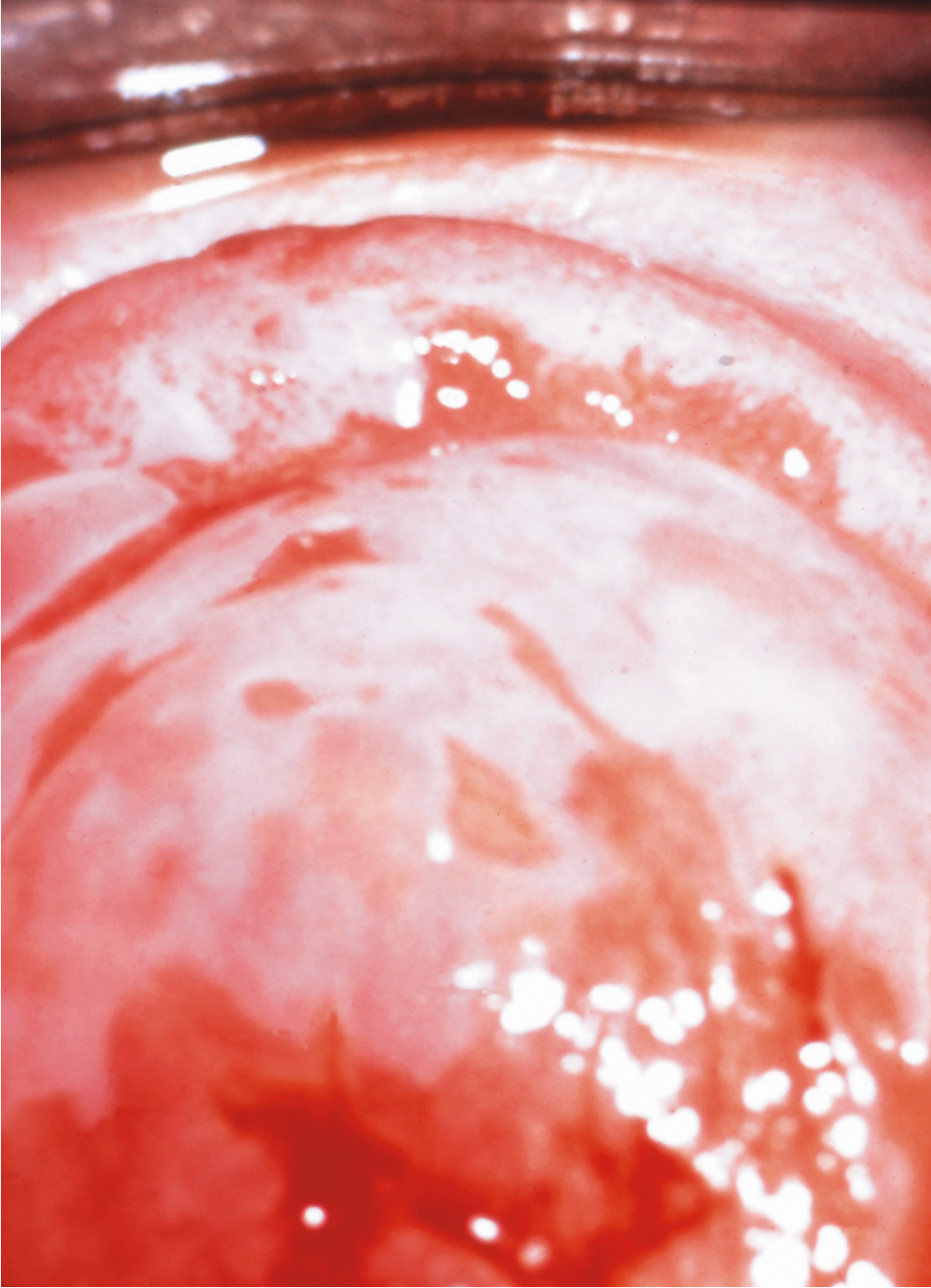
Over time, pruritus occurs with essentially all lesions, leading to scratching, which can develop into ecchymosis and ulceration ( Fig. 2.5 ). Symptoms evolve to include burning, tearing, and dyspareunia. Studies have suggested that the epithelium in lichen sclerosus is metabolically active and non-atrophic. A chronic inflammatory, lymphocyte-mediated dermatosis is present. The etiology of the disease is poorly understood, but autoimmune mechanisms appear to be involved. Among women with lichen sclerosus as compared to those without the disease, there is an increased likelihood of thyroid dysfunction.
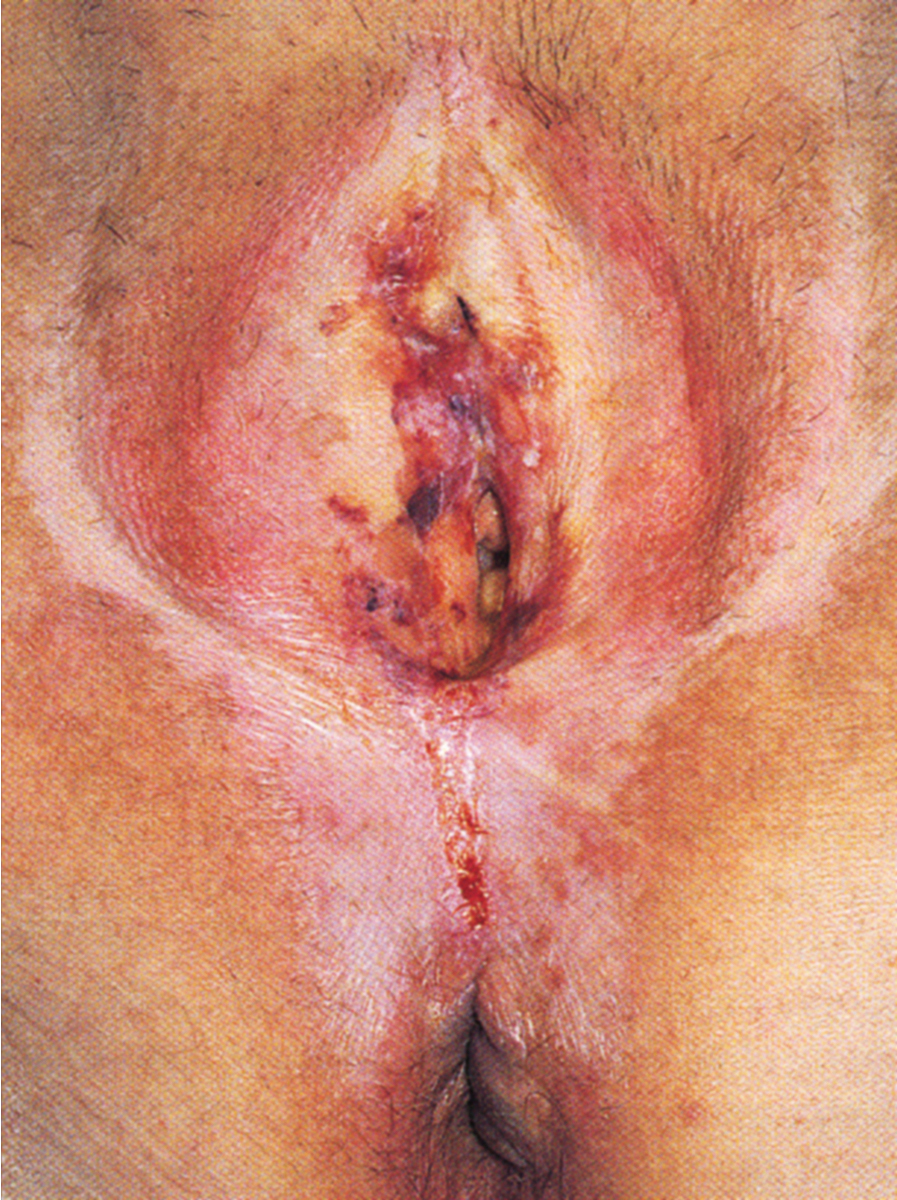
The microscopic features of lichen sclerosus include hyperkeratosis, epithelial thickening with flattening of the rete pegs, cytoplasmic vacuolization of the basal layer of cells, and follicular plugging. Beneath the epidermis is a zone of homogenized, pink-staining, collagenous-appearing tissue that is relatively acellular. Edema is occasionally seen in this area. Elastic fibers are absent. Immediately below this zone lies a band of inflammatory cells that is consistent with lymphocytes and some plasma cells. Lichen sclerosus is often associated with foci of both hyperplastic epithelium and thin epithelium. Lichen simplex chronicus has been found in 27% to 35% of women with lichen sclerosus after microscopic study of vulvar specimens, likely secondary to pruritus and scratching.
In a well-developed classic lesion, the skin of the vulva is crinkled (“cigarette paper”) and thinned, or appears parchment-like. The process often extends around the anal region in a figure-of-8 or keyhole configuration, and 30% of women have perianal lesions. At other times, the changes are localized, especially in the periclitoral area or the perineum. Clitoral involvement is usually associated with edema of the foreskin, which may obscure the glans clitoris. Phimosis of the clitoris is often seen late in the course of the disease. As the disease progresses, there is loss of architecture of the normal external genitalia ( Fig. 2.6 ). The labia minora may completely disappear as a result of atrophy. Synechiae often develop between the edges of the skin in these locations, causing pain and limited physical activity. Fissures also develop in the natural folds of the skin and especially in the posterior fourchette. The introitus may become so strictured or stenosed that intercourse is impossible. The urethra may also become obstructed ( Fig. 2.7 ). The vaginal mucosa is generally spared in this disease, which is helpful in discriminating between lichen sclerosus and lichen planus. In a study by Dalziel, 44 women with lichen sclerosus were evaluated for sexual dysfunction. Apareunia was experienced by 19 of the women at some point. Dyspareunia and decreased frequency of intercourse were noted by 80%, and orgasm was altered and relationships were affected in 50%. Local steroids improved sexual function in two-thirds of these patients. In a recent publication by Kohn and colleagues, approximately 40% of women receiving care in gynecology, vulvar, and dermatology clinics for lichen sclerosus were not sexually active because of lichen and approximately 40% of those women became sexually active again after using topical steroids.
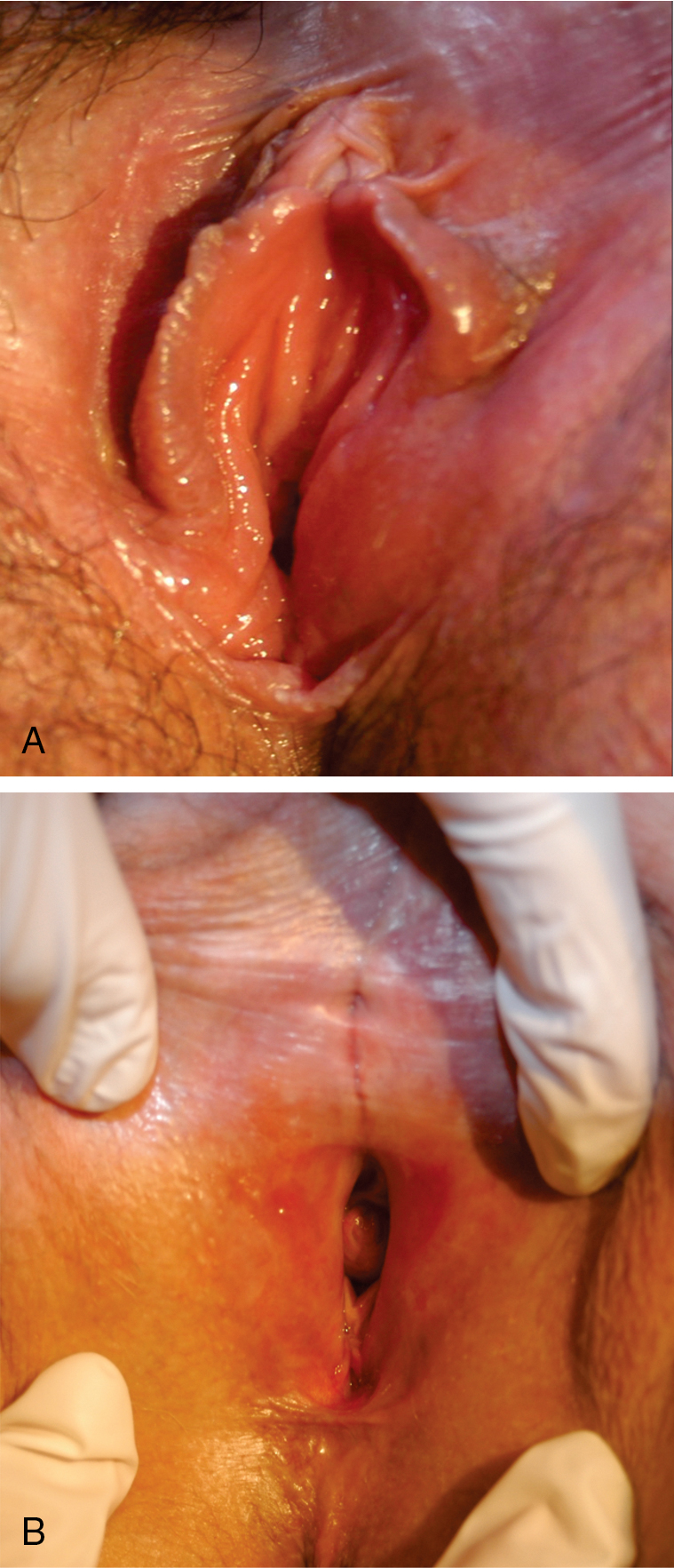
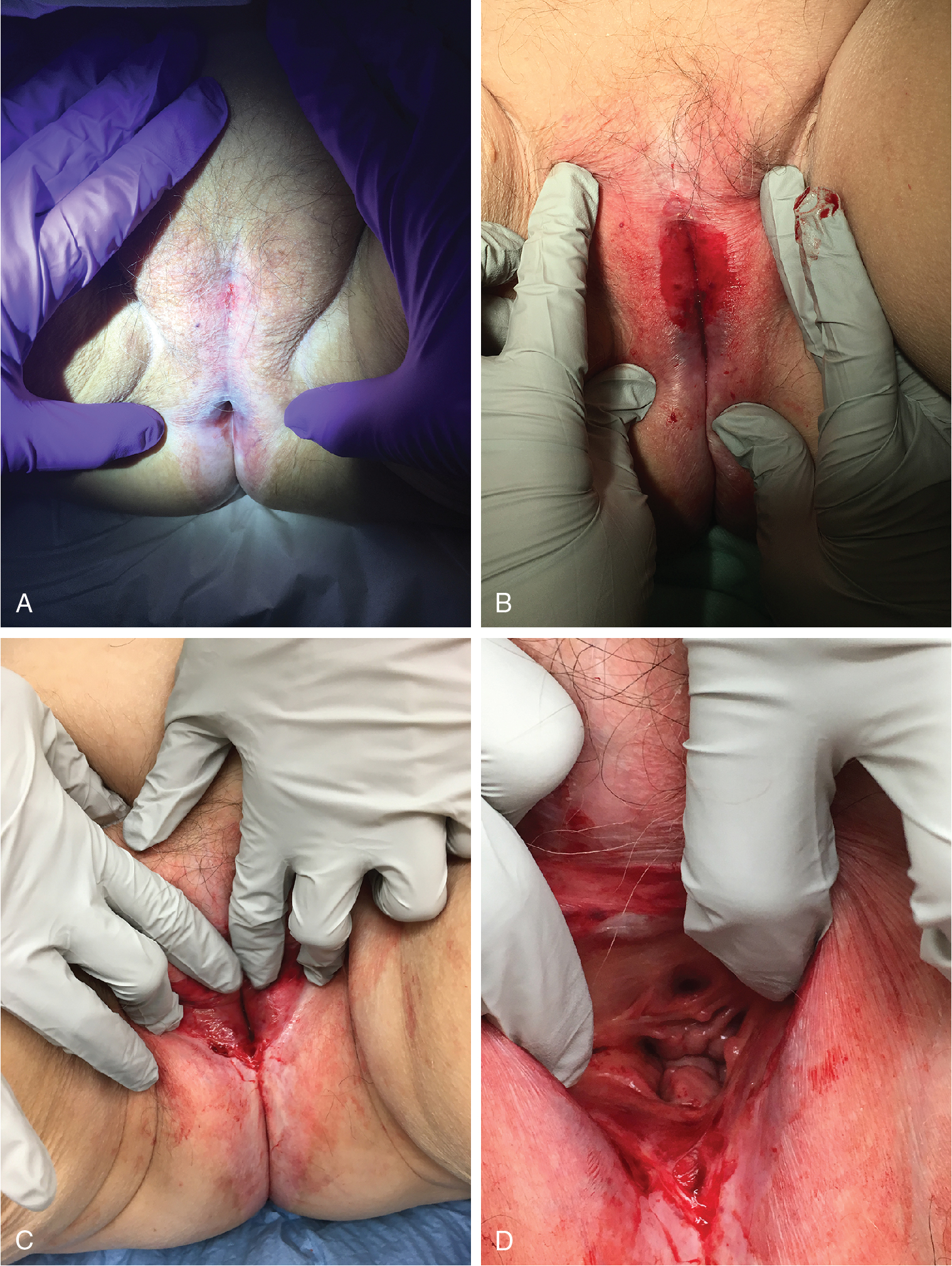
Lichen sclerosus is a risk factor for invasive vulvar cancer, likely as a result of chronic inflammation and sclerosis. Wallace followed 290 women with lichen sclerosus for an average of 12.5 years and found that 4% ( n = 12) developed vulvar cancer. Carlson et al. reported that 4.5% of patients with lichen sclerosus developed vulvar cancer over a mean of 10 years. In treated populations, however, the risk may be lower. Cooper and associates treated 233 women in a vulvar teaching clinic over a 10-year period; 89% of the cohort received superpotent steroid treatment. Invasive vulvar cancer developed in 3% ( n = 7), and vaginal intraepithelial neoplasia (VAIN) developed in 2% ( n = 4). Jones and colleagues reported one case of cancer from a vulvar clinic treating 213 girls and women older than age 8 years. Renaud-Vilmer et al. reported results from an urban dermatology clinic caring for 83 girls and women with no prior treatment of vulvar lichen sclerosus. Invasive cancer was present at the time of presentation in 7% ( n = 6) and developed in 2% ( n = 2); in those who developed cancer, one did not return for follow-up, and one did not use the prescribed treatment. In a cohort followed prospectively with repeat vulvar examinations at the University of Florence by Carli and associates, two cases of invasive cancer and one case of vulvar intraepithelial neoplasia (VIN) developed among 211 women with treated lichen sclerosus. All three cases occurred after more than 3 years of follow-up in the cohort.
The absolute incidence of vulvar cancer is low in women with lichen sclerosus, but 50% to 70% of squamous cell vulvar cancers occur in a background of lichen sclerosus. Currently, there is no diagnostic tool to differentiate between lichen sclerosus that will remain benign versus lichen sclerosus that will evolve into squamous cell carcinoma (SCC). Two biomarkers, p53 and monoclonal antibody MIB1, have shown promise in retrospective tissues studies, but further testing is necessary. The standard of care for cancer screening in this population remains serial examination with directed biopsies for new, evolving, or suspicious lesions, teaching self-examination with a mirror, and self-reporting to a physician when lesions change visibly and to palpation.
Lichen planus
Lichen planus is a distinct dermatologic disease that may affect the oral mucosa, esophageal mucosa, skin, scalp, nails, and eyes in addition to the vulva. The three subtypes of lichen planus that appear to have similar clinical characteristics, but different pathologic appearances, are classic, erosive, and hypertrophic. Approximately 1% of the population likely develops lichen planus; 25% to 50% of women with lichen planus are believed to have vulvar symptoms. Both vulvovaginal-gingival syndrome and penile-gingival syndrome have been described. The disease appears to be a cell-mediated immune disorder causing chronic inflammation. Similar to lichen sclerosus, symptoms are most common in postmenopausal women in their 50s and 60s; usually include pruritus; and can evolve as the disease progresses to include burning, pain, and dyspareunia. Labial agglutination and loss of architecture of the labia and clitoris may occur. The vaginal mucosa is frequently involved, as opposed to lichen sclerosus, which rarely involves the vagina ( Fig. 2.8 ) .
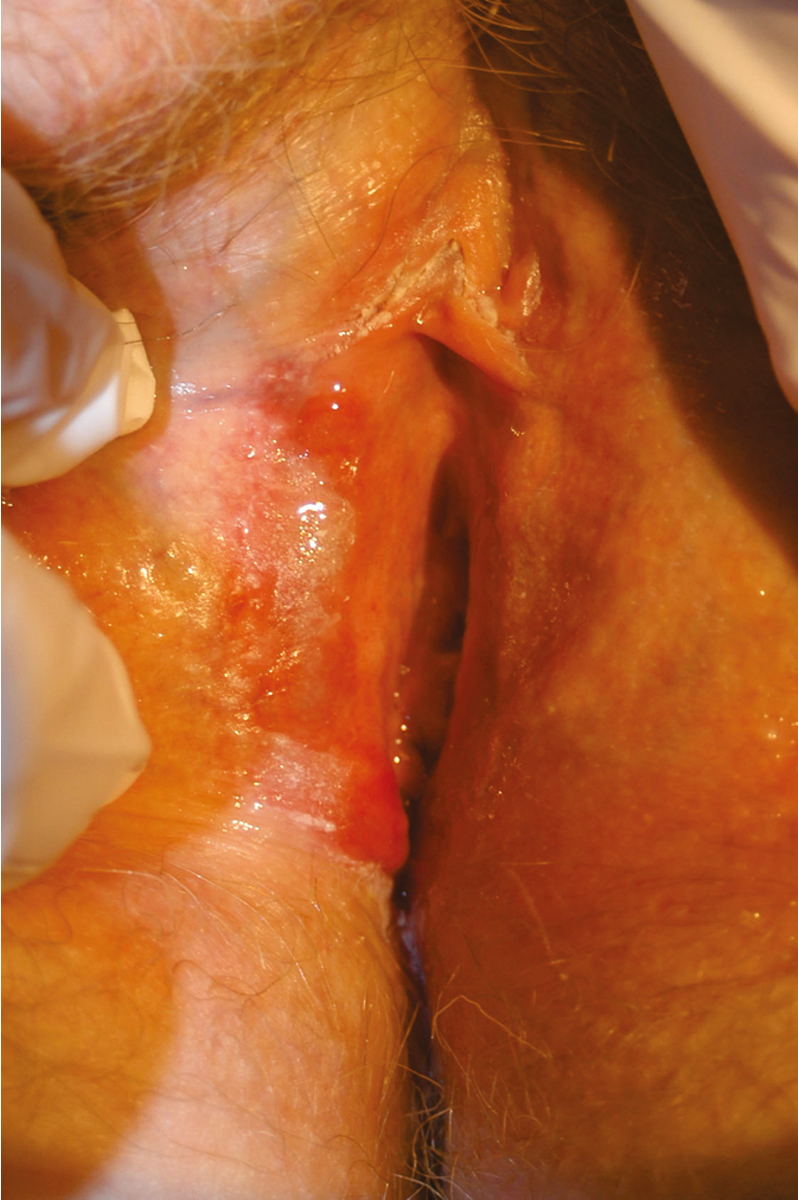
The relationship between lichen planus and vulvar cancer is not as well-established as the association between lichen sclerosus and cancer. Small studies have suggested an increased risk of vulvar cancer, and subjects with oral lichen planus may also have an increased risk of oral cancer. In the early 1990s, case reports by Franck and Young, Lewis and Harrington, and Dwyer and colleagues reported incidences of vulvar SCC in subjects with lichen planus and within erosive lichen planus lesions. In a review of 113 patients with erosive vulvar lichen planus followed by Kennedy and colleagues over an 8-year period, one woman developed vulvar cancer. Of interest, two women in this cohort with oral lichen planus were subsequently diagnosed with oral or esophageal cancer, and two additional women were diagnosed with cervical adenocarcinoma in situ (AIS) and rectal adenocarcinoma. Similar findings were reported by Cooper and associates. Of 114 women with erosive vulvar lichen planus, 7 developed VIN, 1 developed oral SCC, 2 developed anogenital SCCs of the labium minora and perianal area. Because lichen planus can also be a challenging pathologic diagnosis, some of these studies may have mistakenly included women with differentiated VIN (dVIN) rather than lichen planus. Day et al. performed a histopathologic review of a cohort of non–human papilloma virus (non-HPV) associated vulvar cancer and identified lichen sclerosus in 95% of the specimens and dVIN in 88%, with no cases of lichen planus identified. Among those cases with dVIN, 24% resembled erosive lichen planus and 16% resembled hypertrophic lichen planus, highlighting the importance of accurate diagnosis between dVIN and lichen planus. Overall, this study also highlights the overall low contribution of lichen planus to non-HPV vulvar cancer cases.
Pigmented lesions
Pigmented lesions of the vulva are usually intraepithelial, with the exception of melanoma. Pigmented lesions account for approximately 10% of all vulvar disease. The most common pigmented lesion is a lentigo, which is a concentration of melanocytes in the basal layer of cells. It can have the clinical appearance of a freckle, although it is more commonly confused with a nevus. The borders are fuzzy, but it is not a raised lesion. A lentigo is benign, and the diagnosis is usually made by inspection with magnification. If there is any doubt, a biopsy should always be performed as intraepithelial neoplasia of the vulva may appear as a pigmented lesion. Additionally, characteristic raised, hyperkeratotic pigmented lesions are suggestive of carcinoma in situ (CIS) and precursor lesions and should be biopsied.
The management of nevi, however, can be conservative once diagnosed. A nevus can often be detected only microscopically. Unfortunately, a simple nevus and an early melanoma cannot be differentiated on clinical evaluation. Excisional biopsy of these raised, smooth, pigmented areas can be done easily in the physician’s office. If the nevus changes in color, size, and shape, it should be removed for diagnostic purposes. After a nevus is removed, no further therapy is needed regardless of whether it is a compound, intradermal, or junctional type.
Diagnosis and treatment
Biopsies are critical to appropriate management; before any treatment is given on a long-term basis, biopsies should be performed from representative areas to ensure the correct diagnosis. (The exception for biopsy is the pediatric population, which will not be discussed here.) Site selection should be focused on sites of fissuring, ulceration, induration, and thick plaques. Patient education regarding hygienic measures for keeping the vulva clean and dry is important; any soaps, perfumes, deodorizers, or other contact irritants should be avoided. A thorough history should be taken to evaluate possible causes of vulvar pruritus. After lesions with malignant potential have been ruled out, local measures for control of symptoms, primarily pruritus, can be instituted ( Table 2.3 ). Additionally, infectious vaginitis should be ruled out with a saline wet preparation, potassium hydroxide (KOH) wet preparation, and yeast culture to evaluate for atypical yeast that may be missed on plain microscopy. The psychosocial effect should also be considered as many women will feel isolated and have significant detrimental effects in self-perception and intimate relationships.
| Disorder | Treatment |
|---|---|
| Lichen sclerosus | Topical high-potency steroid ointment |
| Lichen planus | Topical high-potency steroid ointment |
| Lichen simplex chronicus | Topical corticosteroid after evaluation and removal of offending environmental agents and treatment of concurrent vaginitis, lichen sclerosus, or lichen planus |
Lichen simplex chronicus
Therapy for lichen simplex chronicus is treatment of the underlying cause of irritation and pruritus. All offending environmental agents should be avoided, including wipes, lubricants, sanitary and incontinence pads, detergents, perfumes, and soaps. A thorough history is often necessary to identify possible sources of irritation. Any underlying vaginitis also requires treatment. For isolated lichen simplex chronicus, a topical steroid ointment such as triamcinolone 0.1% or hydrocortisone 2.5% can be applied daily (after bathing to help seal in moisture) until symptoms are improved, generally in 3 to 4 weeks. If symptoms are particularly severe or a course of low-dose steroids is insufficient, a trial of higher-dose steroid ointment is acceptable. Pruritus is often most severe at night, and short-term use of a pharmacologic sleep aid may be necessary. Lichen simplex chronicus should resolve with removal of the offending agent and treatment; if symptoms persist or recur, repeat biopsies and reconsider the diagnosis.
Lichen sclerosus
Lichen sclerosus is a chronic condition with no curative treatment; the goal is symptom control. The standard treatment for lichen sclerosus is a topical corticosteroid. Typical regimens begin with a superpotent steroid ointment (i.e., clobetasol propionate ointment 0.05%) used nightly until resolution of symptoms (usually 8 to 12 weeks). Because of the chronicity of lichen sclerosus, maintenance therapy is typically necessary after initial treatment; application one to three times per week is usually sufficient. Many women stop treatment when their symptoms improve and then represent months later with recurrent symptoms. Therapy can be reinitiated at treatment levels for 6 to 12 weeks, and emphasis given to maintenance therapy. Theoretically, long-term use of topical steroids may result in striae and thinning of the dermis, but this is infrequently observed in patients with lichen sclerosus. Topical testosterone is no longer the treatment of choice for lichen sclerosus.
A prospective randomized study by Bracco and associates evaluated 79 patients with lichen sclerosus using four different treatment regimens: a 3-month course of testosterone (2%), progesterone (2%), clobetasol propionate (0.05%), and a cream base preparation. Patients experienced greater relief of symptoms with clobetasol (75%) than with testosterone (20%) or other preparations (10%). Clobetasol therapy was the only treatment in which the gross and histologic evaluation of patients improved after treatment. Recurrences after stopping the steroid occurred, but symptoms were relieved when therapy was resumed. Clobetasol was also more effective than testosterone in the randomized trial performed by Bornstein and colleagues, with a significant improvement in long-term relief experience by clobetasol users. Lorenz and colleagues reported 77% had complete remission of symptoms with clobetasol therapy but again noted that maintenance therapy was needed after baseline treatment. In the cohort followed by Cooper and associates discussed previously, 65% were symptom free, 31% had a partial response, and 5% had a poor response after steroid use. Improvement was also seen on physical examination; 23% had total resolution, 69% had partial resolution (improvement in purpura, hyperkeratosis, fissures, and erosions but no change in color and texture), 6% had minor resolution, and 2% had no improvement.
Occasionally, vulvar pruritus is so persistent that it cannot be relieved by topical measures. Topical treatment may also fail if significant hyperkeratosis is present. In such cases, intradermal and lesional injection of steroids has been reported to be effective. If lesions persist and symptoms do not improve after a course of superpotent topical steroids, biopsies should be repeated to confirm the initial diagnosis. It is again critical to rule out cancer and VIN to ensure appropriate treatment. Other regimens have been reported for lichen sclerosus recalcitrant to corticosteroids. Calcineurin inhibitors, such as tacrolimus and pimecrolimus, show some benefit through immunomodulation but are considered second-line therapy. Laser and photodynamic therapy may have some efficacy, but further trials are necessary as initial data has been inconclusive regarding the benefit of these treatments.
As further investigation clarifies the complex pathophysiology and etiology of lichen sclerosus, additional targets for treatment will also be elucidated. Specifically, the understanding of the autoimmune nature of the disease with increase in inflammatory cytokines and activation of the T helper response is promising for further development of treatment options.
Lichen planus
Lichen planus is similarly treated with complete evaluation, patient education, and topical steroids. Additionally, the physical examination should include evaluation for the presence of lichen planus on the skin, scalp, nail beds, and oral mucosa. If systemic disease is present, consultation with a dermatologist is useful. Treatment of vulvar lesions should begin with an ultrapotent steroid ointment (i.e., clobetasol propionate ointment 0.05%) applied nightly for 6 to 8 weeks. If symptoms improve, the frequency of application can be reduced to two to three times weekly for 4 to 8 additional weeks. Cooper and associates reported that in a cohort of 114 women followed and treated for lichen planus, 71% of those treated with ultrapotent topical corticosteroids experienced relief of symptoms with treatment. On examination, 50% experienced healing of erosions, but no patients had resolution of scarring. When lichen planus symptoms are in a prolonged remission, the lowest effective dose is used for maintenance therapy, which may involve less frequent administration and a lower-potency steroid. Similar to lichen sclerosus, symptoms will return if maintenance therapy is not used.
Lichen planus does appear more resistant to therapy than lichen sclerosus. In a small series of women with vulvar lichen planus that was nonresponsive to other treatments, Byrd and colleagues at the Mayo Clinic reported that 15 of 16 subjects experienced symptomatic relief after a course of topical tacrolimus. The mean response time was 4 weeks, and six subjects experienced mild irritation, burning, or tingling that resolved with persistent use. Tacrolimus therapy was less successful in the subjects followed by Cooper and associates, who were nonresponsive to topical steroid treatment. Of seven patients treated, two had complete symptomatic relief, three had some relief, and two had no improvement in symptoms.
Vulvar intraepithelial neoplasia
Terminology
Vulvar pathologic terminology has evolved over the past two decades and clear communication of results and definitions is important for appropriate clinical care. For this reason, many pathology reports include historic terminology in addition to current terminology. Furthermore, current pathology diagnoses are not reflected in the ICD10 system which further inhibits analysis of data, clinical communication, and understanding. Because of the rapid changes, it is also important for today’s clinicians to understand the recent historical terminology in order to interpret medical evidence.
In 2004, the ISSVD clarified the VIN classification system ( Table 2.4 ) and created two distinct VIN categories: VIN usual (uVIN) and VIN differentiated (dVIN), eliminating VIN 1 from the traditional VIN 1, 2, and 3 system as there was no evidence that VIN 1 was a precancerous lesion. The two distinct subtypes of VIN, uVIN and dVIN, were recognized for their differences in epidemiology, morphology, and association with vulvar cancer ( Table 2.5 ). Differentiated type VIN tends to: occur in older women, be unifocal and unicentric, be found at the edge of vulvar SCC and in the setting of lichen sclerosus or planus, and be less frequently associated with HPV. On the other hand, uVIN was identified as being more likely to be found in younger women, have a strong association with cigarette smoking, be multifocal, be less frequently found in conjunction with squamous cell cancer, and usually associated with HPV. Biomarkers also help differentiate uVIN and dVIN, with p16 positivity noted with most uVIN and p53 mutation noted in dVIN.
|
|
|
| Low Grade Squamous Intraepithelial Lesion (vulvar LSIL, previously VIN 1, flat condyloma, or HPV effect) | High Grade Squamous Intraepithelial Lesion (vulvar HSIL, previously VIN Usual Type, VIN2 or VIN 3) | Differentiated VIN (dVIN) | |
|---|---|---|---|
| Age | Premenopausal women (30s–40s) | Premenopausal women (30s–40s) | Postmenopausal women (65 years) |
| Overall % of high grade SILs | N/A | ≈95 | ≈5 |
| HPV associated | Yes | Yes | No |
| HPV type | 6, 11 | 16 | N/A |
| Risk factors | Smoking, immunosuppression | Smoking, immunosuppression | None identified |
| Distribution | Multifocal | Multifocal | Unifocal, unicentric |
| Background histology | Lichen Sclerosus | ||
| Progression to | Condyloma | Warty, basaloid squamous cell cancer | Keratinizing squamous cell cancer |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree