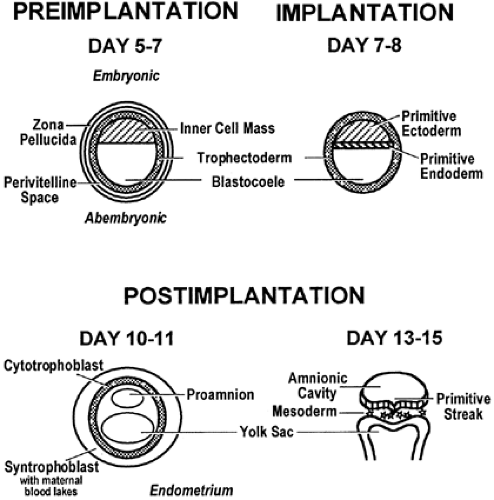
PREIMPLANTATION DEVELOPMENT
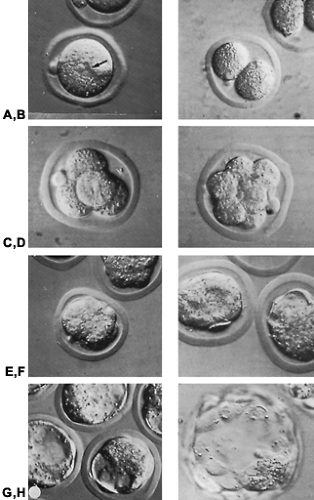
The preimplantation period of development in mammals, the time from conception to nidation (implantation), has variable lengths in the various species. In humans, the preimplantation period lasts for ˜7 days; in the mouse, it is 4 days, whereas in the rat, it is 5 days. The fertilized egg (Fig. 107-3 and Fig. 107-4) is morphologically similar to the mature unfertilized egg. The embryo at this stage is 100 μm in diameter, is associated with two polar bodies remaining from meiotic division, and is surrounded by the amorphous zona pellucida. The zona pellucida, which is composed primarily of three complex glycoproteins known as ZP1, ZP2, and ZP3, is important to early development for several, largely mechanical, reasons. First, there is evidence suggesting that certain glycoproteins of the zona pellucida may play a role in the recognition of the egg by the sperm. Competitive inhibition assays have shown that by incubating mouse sperm with ZP3 before fertilization, binding of the sperm to the zona pellucida is inhibited, thus suggesting that this glycoprotein is responsible for the recognition and binding of the sperm to the zona pellucida.15 Second, the zona responds nearly instantaneously to sperm penetration and renders the egg impervious to additional penetration. Third, the zona provides a constraint to cleavage and ensures that as the
blastomeres divide, they remain together and in the proper orientation. Finally, the zona prevents the naturally sticky cleavage-stage embryo from adhering to the wall of the oviduct as it progresses to the uterus.
blastomeres divide, they remain together and in the proper orientation. Finally, the zona prevents the naturally sticky cleavage-stage embryo from adhering to the wall of the oviduct as it progresses to the uterus.
The mammalian oviduct also plays an important role in these early stages of preimplantation development. Not only does it provide a route through which the embryo is transported from the ovary to its site of implantation in the uterus, but it also provides a crucial timing mechanism for a process known as cleavage division.33,34 and 35 At this stage of development, the embryo divides symmetrically and reductively such that a geometric increase in the number of cells (blastomeres) occurs without an actual increase in the overall size of the embryo. These divisions occur entirely in the oviduct while the embryo is being propelled through its length as a result of ciliary action and muscular contractions in the oviduct wall. The primary function of development at this stage is to provide additional cells and membrane.
Beginning after the first division in the mouse embryo and after the second division in the human embryo, a critical transition occurs in the genetic control of development.36 Before this time, the embryo contains a host of maternally derived mRNAs, ribosomes, and macromolecules that are sufficient to drive transcription and translation through the first (or second, as in the human embryo) cleavage division.37 Further development, however, is dependent on the activation of embryonic control of transcription and the subsequent degradation of maternal mRNAs and proteins.37 In the mouse, one population of polypeptides exhibits at least a two-fold decrease in abundance during the two-cell stage, whereas another population of polypeptides exhibits a similar increase in abundance.38 Although this change most likely reflects the degradation of maternal mRNA and the appearance of new embryonic mRNA, it is possible that this transition is not complete and that some maternal products still may persist for a time after this transition.37
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree