PRECOCIOUS PSEUDOPUBERTY
ETIOLOGY AND CLINICAL CHARACTERISTICS
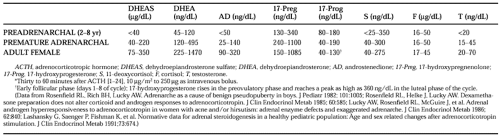
Adrenarche is an “adrenal puberty” during which the adrenal cortex acquires the ability to secrete 17-ketosteroids (see Table 83-2) and DHEA responsiveness to ACTH increases75,76 and 77 (Table 83-3). It normally commences slowly in midchildhood, eventually accounting for most DHEAS production, and reaches a magnitude sufficient to bring about the growth of pubic hair at an average age of ˜12 years.
Adrenarche is independent of true (gonadotropin-mediated) puberty. It is related to the development of the zona reticularis. DHEA synthesis increases because this zone has low 3β-hydroxysteroid dehydrogenase activity and seemingly high 17,20-lyase activity; DHEAS is formed from DHEA because of the high sulfokinase activity of this zone. The existence of a putative pituitary adrenocortical androgen-stimulating factor, which is responsible for adrenarche, has been argued. However, ACTH is necessary and sufficient to stimulate adrenal androgens and is the only hormone established to do so. The authors’ concept is that an adrenarche factor need be responsible only for bringing about the development of the zona reticularis or a shift in its pattern of response to ACTH.
Premature pubarche (sexual pubic hair as an isolated phenomenon before 8.5 years of age in girls or 9.5 years in boys) usually is a benign condition. It has been termed “premature adrenarche” when the plasma DHEAS and testosterone levels are elevated for age, but appropriate for the pubic hair stage. Premature adrenarche is more common in girls than boys. Neither a growth spurt nor clitoromegaly occurs. There are no signs of gonadal maturation, and the bone age is not advanced to an abnormal extent. It has been thought to be a normal phenomenon happening early. However, the androgen level, particularly that of androstenedione, in childhood correlates with the emergence of a polycystic ovary syndrome (PCOS)–like form of functional ovarian hyperandrogenism after menarche.78 This suggests that some cases are due to dysregulation of androgen production, which seems to underlie the primary functional adrenal hyperandrogenism (formerly termed exaggerated adrenarche) that accounts for most cases of postmenarcheal adrenal androgen excess, as well as the frequently associated ovarian androgen excess.77 Although such patients were originally thought to have a mild 3β-hydroxysteroid dehydrogenase deficiency, this is now known to be unlikely unless 17-hydroxypregnenolone responses to ACTH are 7 or more SD above average.79
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree