Definition
The retroperitoneal space, or area, or just “retroperitoneum” is the region or space behind the peritoneal cavity. To be more anatomically correct, the retroperitoneum is the area of the posterior abdominal wall which is located between the posterior parietal peritoneum and the deep lamina of the transversalis fascia. This space is a vast territory lacking an accurate knowledge and accepted map. We, anatomists, need more work to uncover and understand this vast hidden area, this uncharted sea.
Embryologically and anatomically, the retroperitoneal space is related to the thorax above and the lower extremities below. This anatomic highway is responsible for the dissemination of any pathologic entity originatingin the retroperitoneal space. The retroperitoneal space is also closely related to the intraperitoneal anatomical entities. Therefore, traumatic, benign and malignant, and local or diffuse inflammatory processes may spread upward or downward in the peritoneal cavity with connective tissue the pathway of these pathologic entities. An excellent example is the fluid of pancreatitis which can travel practically everywhere: up in the thorax or as low as the triangle of Scarpa.
The peculiar and poorly understood lymphatic pathways and blood vessels are also responsible for the metastatic process of malignant disease of the retroperitoneal space. For all practical purposes, the pericadial space and tunica vaginalis are the only two spaces isolated from the overall continuity of the space-at-large formed by the peritoneal and pleural spaces.
 Embryogenesis
Embryogenesis
The formation of the primitive coelom takes place at the end of the third week or the beginning of the fourth week of embryonic life. Around the third week, the intraembryonic mesoderm is responsible for the differentiation which causes the formation of the lateral plate, the intermediate mesoderm, and the paraxial mesoderm. Further differentiation of the somatic and splanchnic mesoderm divides the lateral plate into layers. Between the somatic and splanchnic mesoderm, on either side of the midline, is the intraembryonic coelom. The right and left intraembryonic coelom unite, forming a single large cavity, which later subdivides to form the pleural, pericardial, and peritoneal cavities and the tunica vaginalis.
At the time of somite formation early in the fourth week, clefts appear in the embryonic mesoderm, occupying a horseshoe-shaped area lateral to the neural plate. They pass across the midline, anterior to the developing head fold of the embryo, and coalesce to form a continuous cavity extending backward beside the head to communicate with the coelomic spaces of the rapidly forming myotomes. The bend in the horseshoe represents the future pericardial cavity. The lateral arms represent the pleural canals, and later, the pleural cavities. These lateral prolongations become dorsal by the rotation of the pericardium under the pharynx. They extend above the septum transversum and lie on either side of the foregut. Ventrally, the ventral parietal recesses (two corresponding prolongations) end blindly.
The pericardial cavity is very large, while the future pleural cavities are narrow, leading into a small peritoneal cavity. As the lungs develop, the pleural cavities enlarge faster than the pericardial cavity. The pleural cavities expand cranially into the somatic mesoderm beyond the cranial limits on the pericardium. The portion of the pericardial wall formed by the pleuropericardial membrane enlarges to form the whole of the lateral walls of the adult pericardium with the rest of the pericardium forming from the portions attached to the anterior and posterior mediastinum, the superior mediastinum, and the diaphragm.
The mesodermal cells lining the intraembryonic cavity change to mesothelial cells by further differentiation. The parietal and visceral layers of the serous membranes are produced from these cells. The somatic mesoderm forms the parietal layer of the serous membrane, eventually lining the pleural and peritoneal cavities. The splanchnic mesoderm forms the visceral layer of the serous membrane covering the abdominal and thoracic viscera. The space subjacent to the serous membrane defines the single subserous space. This is the subperitoneal space of the abdomen and pelvis and the subpleural space of the thorax in the adult. Thus, the formation of the omenta, mesenteries, and ligaments is the result of several embryogenetic steps. The gastrointestinal tract is closed, associated, and fixed with the dorsal and ventral mesentery.
 Surgical Anatomy
Surgical Anatomy
 Topography and Relations
Topography and Relations
Within the retroperitoneal space are embryologically related organs which are referred to as the retroperitoneal viscera. These include the adrenals, kidneys, and ureters. There are also numerous vascular and neural structures, including the aorta and its branches, the inferior vena cava and its tributaries, the lymphatics and the lymph nodes, the lumbar plexus with its branches, and the sympathetic trunks.
In addition to the organs and tissues that develop in the retroperitoneum, several other organs attain a secondarily retroperitoneal position in later embryologic development. These include mostof the duodenum, the pancreas, and major portions of the ascending and descending colon.
Within the greater retroperitoneal space, there are also several small spaces or subcompartments. Loose connective tissue and fat surround the anatomic entities, and, to a variable degree, occupy the smaller spaces. The parietal peritoneum is in continuity with the visceral peritoneum and vice versa.
To be more specific, the visceral organs within the retroperitoneal space are: right and left adrenal, right and left kidney, urinary bladder, ascending colon, descending colon, upper 2/3 of the rectum (approximately), proximal vagina, vas deferens (partially), abdominal aorta, iliac arteries, abdominal inferior vena cava and iliac veins.
The following anatomic entities have a partial relation to the retroperitoneal space: distal esophagus (abdominal esophagus), proximal stomach, liver, and spleen.
Retroperitoneal (Extraperitoneal) Connective Tissue
Hinman subdivided the retroperitoneal connective tissue into three layers (strata)2: outer stratum, intermediate stratum, and inner stratum. The outer stratum forms the abdominopelvic fascia which is the transversalis fascia. The intermediate stratum forms the renal fascia (the fascia of Gerota). The inner stratum is merely the peritoneum and the so-called fusion-fascia (Fig. 3.1) present at the junction of “an intraperitoneal organ (the pancreas, the duodenum, or the ascending or descending colon) with the undersurface of the primitive celomic epithelium.”
Hinman further stated that the inner stratum covers the gastrointestinal viscera and its blood supply.2 The intermediate stratum envelops the adrenals, kidneys, ureters, and the vessels and nerves. The outer stratum forms the internal fascia lining the body wall. We would add the thought that the intermediate stratum represents the extraperitoneal connective tissues that are associated with the anterior, lateral, and posterior walls of the abdominopelvic cavity. These tissues are locally condensed, or otherwise specialized in form, in association with distinct organs such as the kidneys.

Fig. 3.1 Distribution of retroperitoneal fasciae (reproduced from Skandalakis JE, Colborn GL, Weidman TA, Foster RS Jr, Kings-north AN, Skandalakis LJ. Surgical Anatomy: The Embryologic and Anatomic Basis of Modern Surgery. Athens, GR: Paschalidis Medical Publication; 2004)3 (view from below).
 Boundaries of the Retroperitoneal Region (Space)
Boundaries of the Retroperitoneal Region (Space)
The possible boundaries of the retroperitoneal region are as follows.
Above: T12 and 12th rib.
Below: Base of the sacrum, the iliac crest, the upper rami of the pubic bones, and the pelvic diaphragm.
Lateral: The vertical line between the erector spinae muscle (sacrospinalis) and the three flat abdominal muscles or to the lateral border of the quadratus lumborum muscle. Pack and Tabah extended the space as follows4: “In a practical sense, we would extend this space more laterally to the tip of the twelfth rib at a line dropped perpendicularly downward to a point on the iliac crest situated approximately at the junction of the anterior half with the posterior half of the iliac crest. This line corresponds with the point at which the transversus abdominis muscle becomes aponeurotic, by which aponeurosis it arises from the tips and borders of the transverse processes of the lumbar vertebrae.”
Medial: Lumbar and sacral vertebrae with the abdominal aorta and inferior vena cava and their branches; the sympathetic chains and nerve plexuses; the lymphatic elements.
Anterior: The anatomic entities related to the anterior wall and parietal peritoneum of the retroperitoneal space are: part of the liver and its bare area, part of the duodenum, part of the ascending colon, part of the descending colon, and much of the pancreas within the lesser sac. The colon, duodenum, and pancreas are intraperitoneal organs during much of their embryologic development but become fixed in their definitive retroperitoneal positions by fusion of their peritoneal investment with the posterior parietal peritoneum. Posterior: The posterior wall of the space is related to several muscles. From lateral to medial, they are: the aponeurosis of the transversalis abdominis; the quadratus lumborum muscle; most medial, the psoas muscle. These three muscles are covered by a relatively tough layer of fascia which is common for the iliopsoas muscles. However, the anterior layer of the thoracodorsal fascia covers the quadratus lumborum. These boundaries form the lumbar area of the retroperitoneal space, which is the home of the right adrenal gland, the right kidney, the ascending colon, the duodenum, the left adrenal gland, the left kidney, and the descending colon.
There are several subdivisions or zones of the retroperitoneal space, such as: (1) Two fossae: lumbar and iliac; (2) Three zones: Zone 1 (centromedial), Zone II (lateral right and left), Zone III (Pelvic).
Nunn et al.5 proposed a realistic anatomic division of the retroperitoneal space into three zones for description and decision-making in the treatment of retroperitoneal injury. These zones, their boundaries, and their contents are as follows: Zone I (centromedial).
- Upper: Diaphragmatic, esophageal, and aortic openings.
- Lower: Sacral promontories.
- Lateral: Psoas muscles.
- Contents.
Abdominal aorta, inferior vena cava, pancreas, duodenum (partial).
Zone II (lateral).
- Upper: Diaphragm.
- Lower: Iliac crests.
- Lateral: Psoas muscles.
- Contents.
Kidneys and their vessels, ureters and their abdominal parts, ascending and descending colon, hepatic and splenic flexures.
Zone III (pelvic).
- Anterior: Space of Retzius.
- Posterior: Sacrum.
- Lateral: Bony pelvis.
- Contents.
Pelvis in toto, pelvic wall, rectosigmoid colon, iliac vessels, urogenital organs (partial).
Falcone et al.6 reported that a moderate to severe Zone I hematoma after blunt injury identified by computed tomography (CT) coupled with either multiple intra-abdominal injuries or a solid viscus score (SVS) greater than 4 indicated a need for urgent exploration.
Trerotola et al.7 reported the following locations of retroperitoneal hematomas secondary to femoral catheterization in 21 patients (seven patients had hematomas in two locations): retroperitoneum (12), peritoneum (3), groin and thigh (8), and abdominal wall (5).
Radiologic evaluation of the retroperitoneum was investigated by Chaffanjon et al.8 They studied the anatomy of cadavers and healthy subjects using magnetic resonance imaging (MRI) and CT. They postulated that the orientation of the retroperitoneal viscera (the pancreas, the adrenal glands, and the kidneys) depends both on individual morphology and the size of the liver. There are two hepatic landmarks for radiologic imaging: the middle hepatic vein and the portal bifurcation They recommend oblique sectional planes be used for retroperitoneal studies.
We recommend the excellent book of MA Meyers, Dynamic Radiology of the Abdomen, a storehouse of embryologic, anatomic, and pathologic information.9 The interested student should also be familiar with the multiple publications of Oliphant and colleagues.10–14
Iliac Fossa
The iliac fossa is lined with peritoneum which covers the extraperitoneal fat. It continues medially to the retroperitoneal space (lumbar area), then downward to the pelvic wall, as well as forward to the anterior abdominal wall. Just behind the fat is the multilaminar iliacus fascia.
The floor of the iliac fossa is the iliacus muscle. The iliohypogastric nerve usually crosses the iliacus fascia from medial to lateral behind the lower portion of the kidney. Other branches of the lumbar plexus pass through the psoas major and lie deep to the iliac fascia as they cross the iliac fossa. The femoral nerve descends in the lateral part of the interval between the psoas and iliacus muscles.
The common and external iliac arteries and veins, running inferiorly around the brim of the true pelvis on the medial surface of the psoas, are covered with a medial extension of the iliacus fascia. Inferiorly, the fascia iliaca contributes to the formation of the iliopubic tract together with the transversalis fascia and the transversus abdominis muscle.
Pelvic Sidewall
The pelvic sidewall may be presented from a surgicoanatomic standpoint as five surgicoanatomic layers associated with specific anatomic entities.
| First layer | Peritoneum, which is associated with several organs of the digestive, urinary, and genital tracts. |
| Second layer | Abdominal aorta and abdominal inferior vena cava. |
| Third layer | Parietal fascia and visceral fascia. |
| Fourth layer | Nerves of the pelvis. |
| Fifth layer | Muscles: piriformis and obturator internus. |
Psoas Muscle
For all practical purposes, the psoas muscle extends from the posterior mediastinum to the thigh. On its way downward, it is closely associated with the perirenal space and, perhaps, with the posterior pararenal space. One of the authors (JES) witnessed a tuberculous psoas abscess pointing at the medial upper thigh just below the inguinal ligament (Scarpa’s femoral triangle).
Abscesses of pyogenic organisms may be formed by a retrocecal perforated appendix, colonic diverticulitis, or Crohn’s disease. Santaella et al.15 stated that an abscess involving the iliopsoas area should be drained surgically and not percutaneously.
The pathway of renal inflammatory processes is from the perirenal space directly to the psoas muscle. Hematomas in the psoas muscle have been reported. Other pathologic processes involving the muscle are malignant neoplasms of the retroperitoneal space. Such a case was presented by Nathanson and Sonnino.16 They excised the tumor and the entire muscle via the retroperitoneal approach without violation of the peritoneal cavity.
 Compartments of the Retroperitoneal Space
Compartments of the Retroperitoneal Space
Three compartments of the retroperitoneal space are related to the kidney.
- Anterior pararenal compartment.
- Posterior pararenal compartment.
- Perirenal compartment.
The renal fascia, a collagenous connective tissue of mesodermal origin enveloping the kidney, is responsible for this compartmentalization.
The fascial layers and the spaces related to the kidney are as follows, from anterior to posterior:
- Peritoneum.
- Anterior pararenal space (with a variable quantity of loose connective tissue and fat).
- Anterior lamina of Gerota’s fascia.
- Perirenal space (the kidney and the ureter; the adrenal in a separate subcompartment; fat).
- Posterior lamina of Gerota’s fascia.
- Posterior pararenal space (usually with a large content of more compact fat).
- Thoracolumbar (lumbodorsal) fascia and the fascia of the psoas muscle.
To generalize, the muscle fascia lining the abdomen is referred to as the transversalis fascia (Fig. 3.2). More specifically, however, the transversalis fascia, which is the fascial lining of the transversus abdominis muscle, is continuous with the subdiaphragmatic fascia above. Medially, it is continuous with the psoas fascia and the thoracolumbar (or lumbodorsal) fascial investment(anterior lamina) of the quadratus lumborum muscle. Below, it is continuous with the fascia of the iliacus muscle and the parietal muscular fascia of the true pelvis.
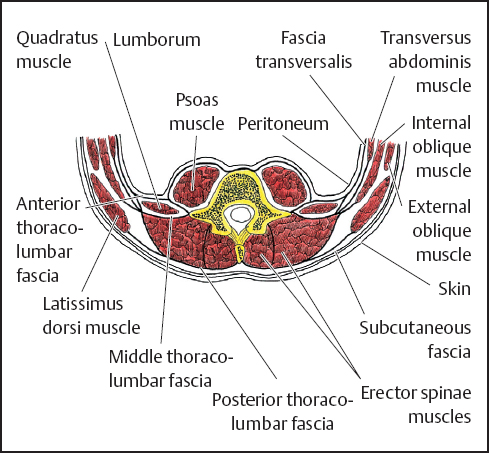
Fig. 3.2 Diagrammatic cross section through posterior body wall in lumbar region.
Perirenal Space
The perirenal space is the home of the kidneys. Therefore, each organ lies between the posterior lamina and the anterior lamina of the fascia of Gerota. Because of the compartmentalization of the retroperitoneal space by the various retroperitoneal connective tissues and fasciae, the kidney is enveloped by the anterior and posterior laminae of the renal fascia (fascia of Gerota or perinephric or perirenal fascia) and by the fatty tissue inside and outside the fascia.
The anterior lamina is also known as the fascia of Toldt and the posterior fascia is known as the fascia of Zuckerkandl.
Last17 called the renal fascia a “vague condensation of the areolar tissue between the parietal peritoneum and the posterior abdominal wall.” However, he added that “certain of its attachments are worthy of note since they serve to restrain the extenuation of a perinephric abscess“ (Fig. 3.3).
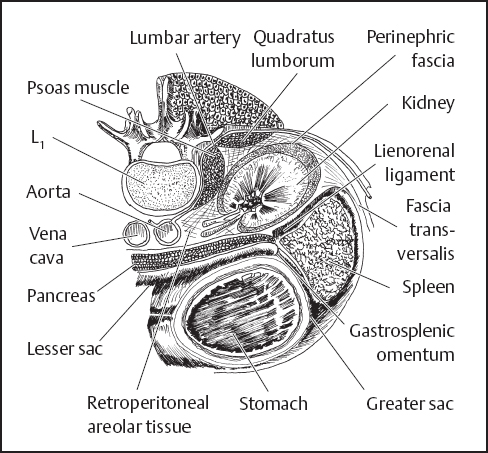
Fig. 3.3 Horizontal section through left kidney, spleen, and stomach.
The renal fascia has a peculiar pathway. It covers the fat of the anterior and posterior surface of the kidney. There is some medial fixation with the adventitial coverings of the renal vessels with extension to the aorta on the left and the inferior vena cava (IVC) on the right. Above and toward the adrenal gland and the diaphragm, the anterior and posterior laminae unite, or perhaps fuse and finally, join the subdiaphragmatic fascia. However, at the upper pole of the kidney, there is a fascial septum separating the adrenal gland from the kidney.
After an anatomic examination of 10 cadavers, Reich et al.18 reported a vascularized, wide-based borderline lamella (corresponding to the anterior lamina of Gerota’s fascia) remaining in the area of the 2nd and 3rd parts of the duodenum. It covered the inferiorvena cava but was fixed with the abdominal aorta.
The lower extent of both the anterior and posterior laminae of the renal fascia is enigmatic. Some anatomists and surgeons believe that the two laminae fuse. However, others believe that they do not fuse, existing in a state of nonunion which thereby permits the kidney alone and not the adrenal gland to travel downward (ptotic kidney, nephroptosis, or floating kidney). Some investigators trying to explain nephroptosis refer to weight loss and, therefore, loss of the perinephric fat which they think keeps the kidney in its normal position. Others support the idea of a weak union which can be ruptured from above by the collection of fluid in the perirenal space downward to the pelvic wall.
Raptopoulos et al.19 reported that perirenal disease does not extend into the pelvis because of the fusion of renal fasciae and the formation of a cone which acts as a barrier to disease extension. The supporters of nonunion theorize that the inferior extensions of the anterior and posterior laminae form the so-called periureteral sheath.
One of the authors (JES) remembers vividly the era of retroperitoneal air insufflation (presacral pneumography). Air was injected into the retroperitoneal space by insertion of a needle anterior to the sacrum and posterolateral to the rectum. This now-discarded procedure, done prior to CT, MRI, etc., precisely outlined the renal contents within the perirenal space. It also indicated pathologic anomalies (if any), especially those of the adrenal glands20 (Fig. 3.4).
In many dissections, the ureter is seen descending from behind the anterior lamina of the sheath, bringing with it a mesentery-like membrane, presumably derived from the perirenal fascia. Downward extension of Gerota’s fascia merges with the pararenal fatty tissue in the infrarenal space.
The posterior renal lamina unites with the anterior renal fascia close to the posterolateral surface of the ascending or descending colon to form the so-called lateroconal fascia. This fascia continues to blend with the parietal peritoneum somewhere at the right or left gutter area anterolaterally. However, it is often seen to be reduced to a thinner mantle of connective tissue intervening between the peritoneum and the transversalis fascia.
Kudos belongs to Meyers and his associates9,10,21 and Parienty et al.22 for their studies of the anatomic and radiologic anatomy of the renal fascia and the compartments it defines (Figs. 3.5 and 3.6). These workers found that both laminae (anterior and posterior) of Gerota’s fascia can be seen radiologically in 50% of patients. The same authors stated that the posterior lamina is thicker in comparison with the anterior lamina. The posterior lamina is also more frequently visualized. The anterior fascia is more prominent on the left side.
The causes of perirenal fluid collection are as follows:
- Bleeding due to blunt abdominal trauma, rupture of a vessel or aortic aneurysm, or spontaneous bleeding secondary to benign or malignant neoplasia.
- Urinoma secondary to obstructive uropathy, blunt abdominal trauma, or iatrogenic injury (secondar to surgery or diagnostic instrumentation).
- Abscess secondary to either pyelonephritis or an infected urinoma.
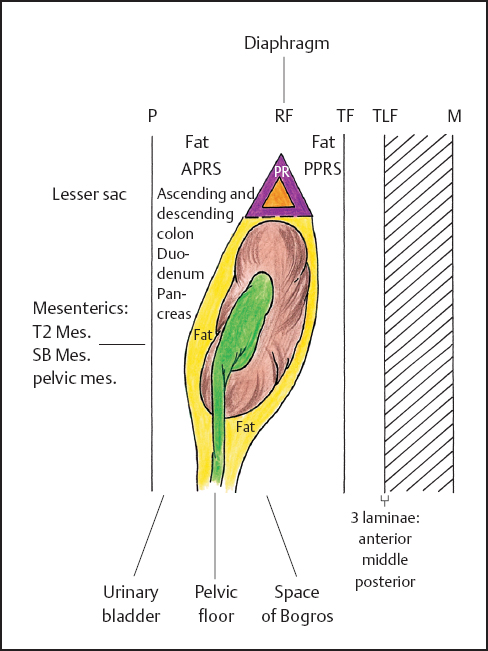
Fig. 3.4 Retroperitoneal spaces (highly diagrammatic). ?, adrenal gland; PR, perirenal space; RF, renal fascia (Gerota‘s); P, peritoneum; APRS, anterior pararenal space; PPRS, posterior pararenal space; TLF, thoracolumbar fascia; TF, transversalis fascia; M, muscles (reproduced from Skandalakis JE, Colborn GL, Weidman TA, Foster RS Jr, Kingsnorth AN, Skandalakis LJ. Surgical Anatomy: The Embryologic and Anatomic Basis of Modern Surgery. Athens, GR: Paschalidis Medical Publication; 2004).
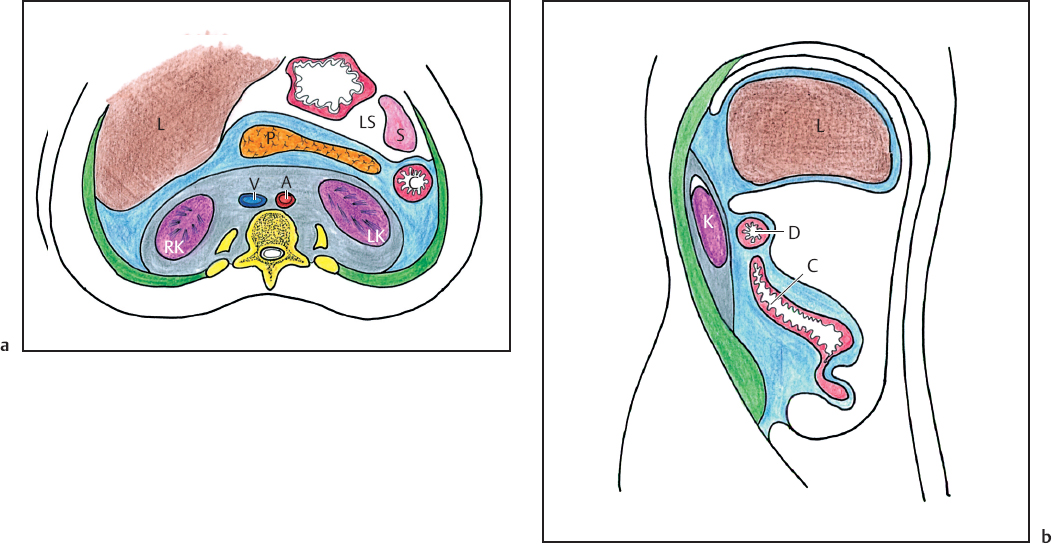
Fig. 3.5a,b a Axial diagram at level of kidneys. b Sagittal diagram in plane of right kidney. Three major retroperitoneal compartments shown: anterior pararenal space (in blue), perirenal space (in grey), posterior pararenal space (in green). L, liver; P, pancreas; LS, lesser sac; S, spleen; V, vena cava; A, aorta; RK, right kidney; LK, left kidney; C, colon; D, duodenum (reproduced from Korobkin M, Silverman PM, Quint LE, Francis IR. CT of the extraperitoneal space: normal anatomy and fluid collections. AJR 1992;159:933–941).25
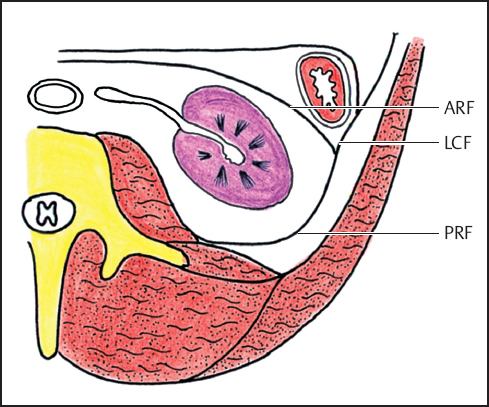
Fig. 3.6 Diagram at the level of the left kidney. ARF, anterior renal fascia; LCF, lateroconal fascia; PRF, posterior renal fascia (reproduced from Korobkin M, Silverman PM, Quint LE, Francis IR. CT of the extraperitoneal space: normal anatomy and fluid collections. AJR 1992;159:933–941).25
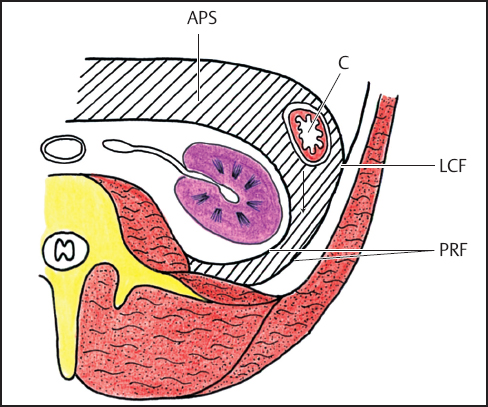
Fig. 3.7 Retrorenal extension of pancreatitis fluid. Diagram shows fluid (hatched area) dissecting layers of posterior renal fascia. APS, anterior pararenal space; C, descending colon; LCF, lateroconal fascia; PRF, two layers of posterior renal fascia (reproduced from Korobkin M, Silverman PM, Quint LE, Francis IR. CT of the extraperitoneal space: normal anatomy and fluid collections. AJR. 1992;159:933–941).25
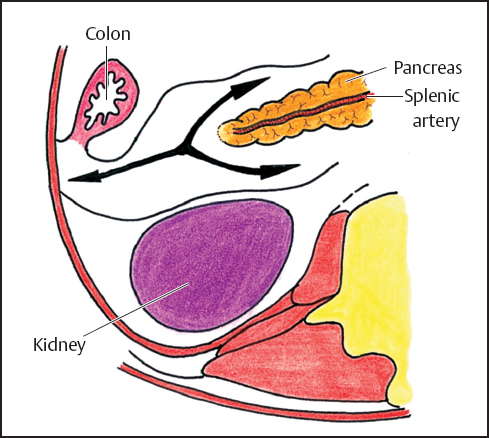
Fig. 3.8 Pathway of extravasations from the tail of the pancreas. (reproduced from Meyers MA. Dynamic Radiology of the Abdomen. 5th ed. New York: Springer; 2000).9
Anterior Pararenal Compartment
We agree with Rubenstein et al.23 that the anterior pararenal compartment (Fig. 3.7) is a distinct retroperitoneal space and not an intraperitoneal space as Dodds et al.24 reported.
According to Korobkin et al.,25 fluid collection is more common on the left side than on the right perhaps due to pancreatitis of the tail (Fig. 3.8). Fluid collection on the right is secondary to duodenal perforation or pancreatitis.
Posterior Pararenal Compartment
Fluid collection in this space is a rare phenomenon. According to Mindell et al.,26 it can occur if sufficient fluid is present.
 Communication of the Retroperitoneal Spaces
Communication of the Retroperitoneal Spaces
The communications of the several retroperitoneal spaces are highly controversial. According to Korobkin et al.,25 the anterior and posterior pararenal spaces merge caudal to the cone of the renal fascia (perhaps conveniently, this area is called the infrarenal retroperitoneal space) which communicates with the prevesical space and other compartments of the pelvic wall.
Raptopoulos et al.27 and Feldberg et al.28 studied the medial attachment of the posterior renal lamina. They reported that the attachment is not always the same, inserting near the anterior or the posterior surfaces of the psoas muscle. According to these authors, the attachment is most likely at the posterior aspect of the psoas muscle, or occasionally, at the quadratus lumborum muscle at the area of the lower renal pole.
The medial extension of the anterior lamina is highly controversial. To what extent does it continue across the midline? If such continuation exists, do right and left perirenal spaces communicate? It is our opinion, from an anatomic standpoint, that communication between the right and left perirenal spaces is somewhat questionable. However, Kneeland et al.29 and Mindell et al.,26 using radiologic and cadaveric studies, reported that the perirenal spaces may communicate across the midline anterior to the aorta and the inferior vena cava (IVC) and posterior to the anterior laminae of the renal fascia. If this is true, a midline vascular pathology, such as an aortic aneurysm, may travel to the left or even to the right perirenal space. Hopper et al.30 disagreed, advising that the pathway of the blood from such a rupture is to the psoas muscle and then into the posterior pararenal space. Last17 may have been correct that this “layer” is just areolar tissue. Raptopoulos et al.27 agree with Last that the anterior lamina is thin.
Since the anterior lamina is thin, perhaps the lateroconal fascia is the product of the posterior lamina only. The water is muddy, the anatomy is confused; perhaps we cannot interpret the radiologic images of the anatomy 100% correctly. In the operating room, many times have surgeons been unsure about the formation of compartments, the pathway of fluids and, finally, the accurate localization of compartmental collection. Maybe Last17 was right. Or, perhaps, the amount of blood (bleeding from injury to the retroperitoneal organs) or inflammatory potentialities (secondary, perhaps, to pancreatitis) play a greater or lesser role in the final picture.
According to Korobkin et al.,25 an abscess or infected fluid can be found in any compartment. Pancreatic fluid secondary to pancreatitis is most likely to collect in the anterior pararenal space, or perhaps, in all retroperitoneal compartments. We agree. As a matter of fact, we (JES, LJS) have seen a case of intraperitoneal pancreatic extravasation presenting as hydrocele secondary to collection at the tunica vaginalis.
Hureau et al. reported the following31,32:
- The anterior pararenal space is almost virtual (quasi virtuel), demonstrating its existence through pathologic manifestations such as acute pancreatitis.
- The posterior pararenal space continues to the space of Bogros (Fig. 3.9) which is a lateral extension of the retropubic space of Retzius. The space of Bogros is formed laterally by the iliac fascia, anteriorly by the transversalis fascia, and medially by the parietal peritoneum.
- The perirenal spaces continue down to the bladder and the prevesical space.
We want to emphasize that we do not have all the answers about these spaces, which extend to the diaphragm and to the pelvis, assuming the guided migration of pathologic processes. However, we must always remember the upward and downward continuation of the retroperitoneal space: thorax and neck as well as pelvis and triangle of Scarpa (Figs. 3.10-3.12).
There are several other spaces and anatomic entities related to the retroperitoneal space, such as the subperitoneal pelvic space (extraperitoneal), the vesicoumbilical fascia and vesical spaces. It is not within the scope of this chapter to present all these spaces.
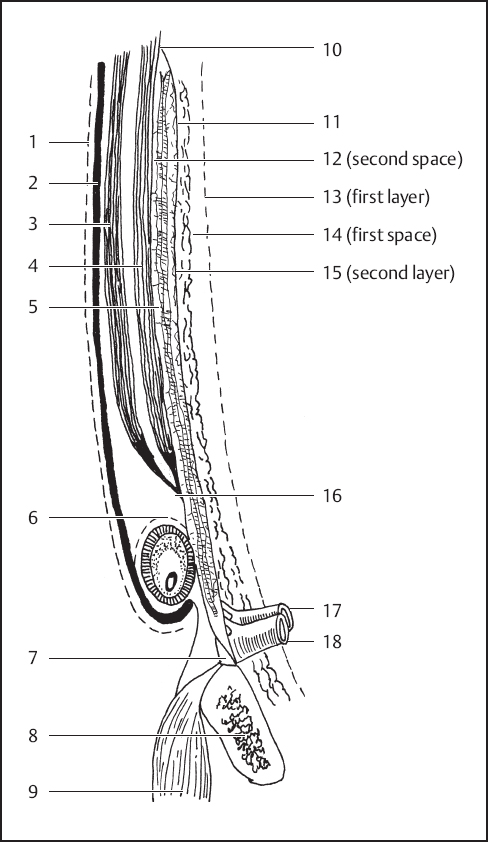
Fig. 3.9 Highly diagrammatic representation of the layers of the abdominal wall and inguinal area. 1, External oblique fascia (fascia of Gallaudet); 2, external oblique aponeurosis; 3, internal oblique muscle; 4, transversus abdominis muscle and its aponeurosis; 5, transversalis fascia anterior lamina (third layer); 6, external spermatic fascia; 7, Cooper’s ligament; 8, pubic bone; 9, pectineus muscle; 10, possible union of transversalis fascia laminae; 11, transversalis fascia posterior lamina (second layer); 12, vessels (second space); 13, peritoneum (first layer); 14, space of Bogros (first space); 15, preperitoneal fat; 16, transversus abdominis aponeurosis and anterior lamina of transversalis fascia; 17, femoral artery; 18, femoral vein (modified from Skandalakis JE, Colborn GL, Androulakis JA, Skandalakis LJ, Pemberton LB. Embryologic and anatomic basis of inguinal herniorrhaphy. Surg Clin North Am. 1993;73:799– 836; with permission. Drawn with R.C. Read).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


