© Springer-Verlag Italia 2015
Raul Mattassi, Dirk A. Loose and Massimo Vaghi (eds.)Hemangiomas and Vascular Malformations10.1007/978-88-470-5673-2_3636. Possibilities and Limits of Medical Treatment
(1)
Department of Angiology, Swiss Cardiovascular Center, Bern, Switzerland
Keywords
Localized intravascular coagulopathyLow molecular weight heparinThrombosisBleedingInfectionMedical management of vascular malformations is focused on prevention of symptoms and complications. There is currently no medical option to cure the vascular malformation in itself, unlike in vascular tumors. Progress, however, has been made in the use of drugs specifically targeting the vessels of the malformations themselves, such as antiangiogenic drugs. These are promising approaches, but not yet in widespread clinical use, and they are discussed at the end of this chapter.
Thrombosis and Bleeding
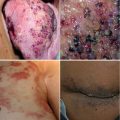
Slow-flow vascular malformations are associated with an increased risk for thrombosis as well as bleeding [1, 2]. The pathogenesis of coagulopathy in vascular malformations is still poorly understood. Thrombocytopenia which can be severe in vascular tumors (e.g., Kasabach-Merritt syndrome in giant hemangiomas) is usually mild, if present at all. However, in extensive venous malformations, ectatic vessels can lead to stasis with activation of the coagulation cascade as one component of the classic Virchow’s triad. Additional factors might be abnormalities of the endothelium within the malformed vessels resulting in a local disruption of the regulation of coagulation, and several studies have been able to demonstrate this localized intravascular coagulopathy (LIC) [3–6]. Laboratory assessments show low levels of fibrinogen and elevated D-dimers. Severity of LIC seems to be related to the extent of the malformation [5]. LIC rarely results in serious complications, but it can be aggravated by different stimuli such as surgery, endovascular therapy, or trauma, resulting in disseminated intravascular coagulopathy (DIC). It is especially important to bear this in mind in the perioperative management of patients with vascular malformations, and diligent prophylaxis with low molecular weight heparin (LMWH) is the recommended [7]. It seems reasonable to assume that large malformed draining veins, such as the marginal vein which is frequently ectatic and typically valveless, can be a source of pulmonary embolism [8, 9]. Elimination of such veins should be considered in patients who have suffered a pulmonary embolism. In general, any extensive venous malformation should be assumed to be a risk factor for venous thromboembolism, and prophylactic measures should be contemplated in any situation with increased risk.
Patients with venous malformations frequently suffer from painful episodes of local thrombosis within the malformed veins. These can often be successfully treated – minimizing intensity and duration of pain – with short courses of LMWH, similar to the treatment of superficial vein thrombosis in patients without vascular malformations [3]. In patients who experience such episodes very frequently and in whom this treatment has proven beneficial, self-administration of LMWH at the onset of symptoms should be discussed. Continuous long-term heparinization can be considered in patients with severe and chronic pain that has responded to LMWH treatment. With the increasingly widespread use of novel oral anticoagulants, an alternative to treat superficial thrombosis might be emerging, although at this point there is very little experience in this setting, and the use of these agents is off-label.
Depending on the site of the malformation, recurrent bleeding can cause chronic anemia. It is often not possible to completely eliminate the lesion to prevent bleeding. Anemia in these patients should be treated the same as chronic anemia by other causes. This might require iron replacement therapy or even regular red blood cell transfusions. As vascular malformations can predispose patients to bleeding as well as thromboembolic events, a particularly challenging clinical problem can occur when there is recurrent bleeding and an indication for anticoagulation, and risk and benefits must be carefully weighed.
Infections
Malformations with a lymphatic component carry a risk of infection. These patients need to be aware of this risk and instructed on how to act appropriately to prevent infection, how to recognize early signs, and when to seek medical attention or, in select cases, initiate self-medication. Preventive measures include good skin care and treatment of possible sources of infection (e.g., athlete’s foot in malformations of the lower extremities). When an infection occurs, prompt treatment with an appropriate antiinfective agent is important. Choice of the specific antibiotic does not differ from similar infections in patients without vascular malformations. If infections are recurring, patients should be given the option to initiate treatment themselves at the first sign of infection. In patients with frequent infections, a long-term antibiotic prophylaxis should be considered.
Medications to Avoid
In extratruncular malformations, oral contraceptives containing estrogen should be used with caution. They can stimulate proliferation of malformed vessels (in particular in arteriovenous malformation), similar to endovascular or surgical trauma, and precipitate worsening of symptoms. Additionally, especially in patients with extensive venous malformations, risk of thromboembolic disease is increased, and this risk could be substantially heightened by the addition of estrogen-containing drugs.
Nonmedical Conservative Treatment
For the large majority of patients with vascular malformation, the most important conservative treatment by far is compression therapy. Adequate compressive treatment can minimize symptoms and prevent complications and progression of the vascular malformation. The specifics of compression therapy do not differ from those for chronic venous insufficiency or lymphedema.
A considerable number of patients with vascular malformations report signs of anxiety, depression, and somatic or psychological distress if specifically prompted [10]. Treating physicians should be aware of the psychological impact that a vascular malformation can have and should offer appropriate support.
Future Developments
Thalidomide has been shown to reduce bleeding in patients with hereditary hemorrhagic telangiectasia, possibly by promoting vessel maturation [11]. Additionally, several reports about positive effects of thalidomide in gastrointestinal bleeding due to vascular malformations have been published [12–14]. The drug is also known to have an antiangiogenic effect by suppression of endothelial growth factor (EVGF) [15]. A combination of thalidomide and interferon-α has been used in extensive CVMs with acceptable results [16]. In another case, the use of a combination of thalidomide, interferon, and zoledronate has been described [17]. The most important adverse effect of thalidomide is peripheral neuropathy which occurs in about 20 % [18, 19]. In summary, the efficacy of this drug in CVM has yet to be demonstrated, and data exists mainly on the effect in gastrointestinal bleeding, but not in other VM, LM, or AVM.
Sildenafil selectively inhibits phosphodiesterase 5, decreasing the contractility of vascular smooth muscle and resulting in vasodilation [20]. The drug has been approved for the treatment of pulmonary hypertension in adults and is used off-label in children for the same pathology [21]. Some positive effect of this drug on complex lymphatic malformations has been reported [22–24]. This effect could be due to smooth muscle relaxation in lymphatic cysts which in turn might facilitate either relaxation of the cysts themselves or draining of the cysts. However, no randomized study exists to assess effectiveness of sildenafil in this setting.
Sirolimus (also known as rapamycin), a macrolide produced by Streptomyces hygroscopicus bacteria, was originally developed as an antifungal agent [25]. After exhibiting immunosuppressive and antiproliferative effects [26], it was then used to prevent kidney transplant rejection [27] and to improve results of coronary stenting by applying it in drug-eluting stents [28]. Additionally, sirolimus demonstrated an anticancer effect by inhibition of angiogenesis [29] and has been utilized with good results in tumors [30, 31]. Recent reports indicate a possible positive effect in the treatment of vascular malformations, mainly of the lymphatic variety [32, 33]. A phase II clinical trial on different types of vascular malformations – mainly lymphatic, but also venous and kaposiform hemangioendothelioma – was presented at the ISSVA (International Society for the Study of Vascular Anomalies) 20th International Workshop in Melbourne, Australia, in 2014. Eighty-two percent of malformations demonstrated a partial response, and 5 % were stable, while a progression was noted in 12 % [34]. Treatment with sirolimus had a positive effect in three cases of Gorham-Stout syndrome [35]. Blue rubber bleb nevus syndrome has been treated successfully in an 8-year-old patient with massive gastrointestinal bleeding. A treatment with low doses of sirolimus completely halted bleeding during the 20-month follow-up and reduced venous masses [36]. Toxicity leading to interstitial pneumonitis and decreased glucose tolerance has been reported in other settings, but so far has not arisen in any reported cases of use of sirolimus in vascular malformation.
Octreotide is a somatostatin analogue which has been used for the treatment of gastrointestinal bleeding due to CVM. The drug may have an antiangiogenic effect [37] in addition to gastrointestinal effects like reduction of gastrin and pepsin and decrease in duodenal and splanchnic blood flow [38, 39]. Some series demonstrate a reduction of necessary transfusions after octreotide therapy [40]. It can be considered as an adjunctive treatment for gastrointestinal bleeding due to CVM or as the sole treatment if other, more aggressive options are not possible. No data exists about the usefulness of octreotide on CVM outside the gastrointestinal tract.
Estrogen and progesterone have been widely investigated in the treatment of gastrointestinal bleeding caused by vascular malformations. This was based on the observation that epistaxis in HHT improved in pregnancy and worsened after menopause [41]. Case reports showed positive results [42, 43]. However, a randomized multicenter study failed to demonstrate an advantage over placebo [44].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree